Partial melting
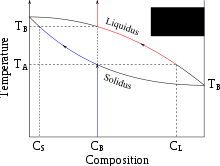
Partial melting occurs when only a portion of a solid is melted. For mixed substances, such as a rock containing several different minerals or a mineral that displays solid solution, this melt can be different from the bulk composition of the solid.
Partial melting occurs where the solidus and liquidus temperatures are different. For single minerals this can happen when they exhibit solid solution, for example in olivines between iron and magnesium. In rocks made up of several different minerals, some will melt at lower temperatures than others.
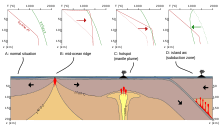
Partial melting is an important consideration in geology with respect to the chemical differentiation of crustal rocks. Virtually all rocks on Earth derive from material from the interior of the earth that has been partially melted. The main places where partial melting occurs are subduction zones, mid-ocean ridges and hotspots. In all these places partial melting is often associated with volcanism, although some melts do not make it to the surface. Partial melts are thought to play an important role in enriching old parts of the continental lithosphere in incompatible elements.[1] Partial melts produced at depth move upwards due to the compaction of the surrounding matrix.[2]
References
- ↑ Gibson, Sally A.; Jacqueline Malarkey; Jason A. Day (2008-10-22). "Melt Depletion and Enrichment beneath the Western Kaapvaal Craton: Evidence from Finsch Peridotite Xenoliths". Journal of Petrology. 49 (10): egn048. doi:10.1093/petrology/egn048. Retrieved 2009-05-22.
- ↑ McKenzie, Dan (1987-04-01). "The compaction of igneous and sedimentary rocks". Journal of the Geological Society. 144 (2): 299–307. doi:10.1144/gsjgs.144.2.0299. Retrieved 2010-01-21.