Parapoxvirus of red deer in New Zealand
| Parapoxvirus of red deer in New Zealand | |
|---|---|
| Virus classification | |
| Group: | Group I (dsDNA) |
| Family: | Poxviridae |
| Subfamily: | Chordopoxvirinae |
| Genus: | Parapoxvirus |
| Species: | Parapoxvirus of red deer in New Zealand |
Parapoxvirus, is commonly referred to as "farmyard pox" and is mostly expressed in hoofed animals. The virus belongs to the Poxviridae family.[1] This infection is identified by scabby lesions that can be seen on the muzzle, lips, face, ears or on the velvet of the Red deer (Cervus elaphus). This virus is zoonotic, meaning infectious diseases of animals that can be transmitted to humans. Humans vulnerable to infection include farmers, butchers, and veterinarians. This virus occurs worldwide.
In 1987, deaths occurred on two Red Deer farms in New Zealand where secondary bacterial infections were seen alongside the lesions. In these particular cases, morbidity rates reached 100%.[2][3]
Viral classification
Parapoxvirus belongs to the family of viruses named Poxviridae, a group one family of double stranded DNA viruses. More specifically Parapoxvirus is classified into the subfamily of Chordopoxvirinae. Other Chordopoxvirinae genera include; Orthopoxvirus, Avipoxvirus, Capripoxvirus, Leporipoxvirus, Suipoxvirus, Molluscipoxvirus, Yatapoxvirus. The second subfamily is Entomopoxvirinae with genera; Alphaentomopoxvirus, Betaentomopoxvirus, and Gammaentopxvirus[4]
Virion size
Generally, viruses within the Poxviridae family have brick or oval-shaped virions. Sizes range from between 140-260 nanometres in width and 220-450 nanometres in length. They are enveloped viruses with surface tubules sometimes referred to as surface filaments.
Structure
Parapoxvirus virions are large in comparison to a majority of the other Poxviridae virions. Virions are the complete form of a virus outside of the host cell that is capable of infection. Virions contain a core of RNA or DNA within a capsid. A capsid is the protein shell of a virus. Parapoxvirus virions are typically 260 x 160 nanometers in size.They possess an enveloped capsid and a distinguishing spiral coat,[1] which is composed of a crossing pattern of tubes. Dissecting a Parapoxvirus virion from the outside in, after passing through the either the EV envelope or the MV membrane (depending on the infectious virus particle, see bullets below) is the lateral body, its function is unknown. After this comes the core wall which is followed by the nucleocapsid.[5] The nucleocapsid is simply the capsid of the Parapoxvirus that is enclosed in nucleic acid. Surprisingly, parapoxviruses have considerably smaller genomes that other genera of the family Poxviridae, (85 MDA).[6]
Parapoxvirus has two different infectious virus particles
- the intracellular mature virus (IMV)
- the extracellular enveloped virus (EEV)[7]
Genome
Parapxvirus is a linear, double-stranded DNA virus. The length of the genome ranges from 130 – 150 kb. "The linear genome is flanked by inverted terminal repeat (ITR) sequences which are covalently-closed at their extremities."[7]
Replication cycle
These viruses can infect ungulates (hoofed animals) and humans. Transmission can be transmitted sexually and non sexually.[6]
Entry into cell
Parapoxvirus enters cells by utilizing glycosaminoglycans as a lubricant, which are "long unbranched polysaccharides consisting of a repeating disaccharide unit. The repeating unit (except for keratan) consists of an amino sugar (N-acetylglucosamineor N-acetylgalactosamine) along with a uronic sugar (glucuronic acid or iduronic acid) or galactose. Glycosaminoglycans are highly polar and attract water."[8]
Replication and transcription
Replication is Cytoplasmic
- Viral proteins attach to the host glycosaminoglycans (GAGs). This brings about endocytosis which allows the virus penetration of the host cell.
- The virion then fuses with the plasma membrane of the host cell and releases its core into the cytoplasm of the host cell.
- Early Phase: In the cytoplasm, early genes are transcribed by viral RNA polymerase. This occurs half an hour after the initial infection.
- Intermediate Phase: Intermediate genes are expressed after the early genes. This triggers genomic DNA replication. Usually 100 minutes post-infection
- Late Phase: Between 140 minutes and 48 hours post-infection, late genes are expressed. All structural proteins have now been completed.
- An immature spherical particle is assembled in cytoplasmic viral factories.
- It then matures into the brick-shaped intracellulare mature virion (IMV)
- IMV virion is released upon cell lysis or it can bad after forming an external enveloped virion (EEV). To do this the IMV virion must receive a second double membrane from the Golgi apparatus.[7]
Assembly and release
Assembly occurs within the cytoplasm of the host cell. Release occurs via budding of a membranous vesicle and ultimately results in lysis. The host cell is denigrated after the cell's membrane is ruptured upon virus' exit.
Interaction with hosts
Parapoxviruses have been seen in a variety of hosts, other than Red Deer of New Zealand, examples include: California Sea Lions, European Musk Oxen, Finnish Reindeer, Harbor Seals in the North Sea, Japanese Serows, Red Squirrels in the United Kingdom, and White-tailed deer in the United States.[1]
Most infected animals express lesions on lips, nostrils, eyes, udders, and/or groin while a select few do not exhibit lesions at all.[1]
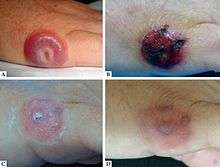
Associated diseases
The viral infection of Parapoxvirus genus is the Paravaccinia virus. The infection will present itself in humans classically as lesions on the skin but it can also cause fever and fatigue which are often overlooked by the infected individuals. Shown in the image. It tends to cause blisters or nodules as well, 4 millimeters in diameter. This is transmitted by contact with infected livestock, such as the Red Deer of New Zealand. It is common within human that are around susceptible species often. This includes veterinarians, ranchers, and cattle milkers.[9] Lesions tend to form between initial contact and three weeks after that contact with an infected Red Deer or other animal. The first chase of Paravaccinia was recorded in 1799 by Edward Jenner. He associated the humans with lesions and their contact with infected livestock.[9]
Treatment and medications
Red deer and other undulate's lesions tend to clear up with little to no scaring between 4 and 8 weeks after infection. There do not appear to be any vaccines present for Parapoxvirus in humans or animals.[10] Veterinarians usually prescribe antibiotics in order to prevent secondary bacterial infections of the lesions. The 1987 outbreaks in New Zealand to Red Deer only lead to death because of such infections without treatment. Surgical removal of lesions increases the healing rates and also prevents fungal or bacterial infections from ultimately killing the animals.[9]
Recent outbreaks
- 1 January 2011: Parapoxvirus, was confirmed in a human, in the United States of America
- 22 August 2006: Parapoxvirus, was confirmed in squirrels, in England
- 22 July 2006: Parapoxvirus, was confirmed in squirrels, in England
- 18 July 2006: Parapoxvirus, was confirmed in squirrels, in England
- 16 July 205: Parapoxvirus, was confirmed in squirrels, in Scotland
- 8 March 2003: Parapoxvirus, was confirmed in squirrels, in England[11]
Comparison to outbreak in Italy
In order to characterize the strains of Parapoxviruses causing severe disease in wild ruminants in Stelvio Park, Italy, sequencing and comparisons of isolated DNA were conducted. Results demonstrated that the red deer isolates are closely related to the parapox of Red Deer in New Zealand virus.[12]
References
- 1 2 3 4 "Parapoxviruses: Background, Pathophysiology, Epidemiology". 2017-09-28.
- ↑ Horner, G. W.; Robinson, A. J.; Hunter, R.; Cox, B. T.; Smith, R. (1987-04-01). "Parapoxvirus infections in New Zealand farmed red deer (Cervus elaphus)". New Zealand Veterinary Journal. 35 (4): 41–45. doi:10.1080/00480169.1987.35376. ISSN 0048-0169. PMID 16031369.
- ↑ Ueda, Norihito; Inder, Marie K.; Wise, Lyn M.; Fleming, Stephen B.; Mercer, Andrew A. (March 2007). "Parapoxvirus of red deer in New Zealand encodes a variant of viral vascular endothelial growth factor". Virus Research. 124 (1–2): 50–58. doi:10.1016/j.virusres.2006.09.012. ISSN 0168-1702. PMID 17109982.
- ↑ "http://viralzone.expasy.org/174?outline=all_by_species". viralzone.expasy.org. Retrieved 2017-11-03. External link in
|title=(help) - ↑ "http://viralzone.expasy.org/150?outline=all_by_species". viralzone.expasy.org. Retrieved 2017-11-03. External link in
|title=(help) - 1 2 "Parapoxvirus - an overview | ScienceDirect Topics". www.sciencedirect.com. Retrieved 2017-10-29.
- 1 2 3 "http://viralzone.expasy.org/150?outline=all_by_species". viralzone.expasy.org. Retrieved 2017-10-29. External link in
|title=(help) - ↑ "Glycosaminoglycan". Wikipedia. 2017-10-26.
- 1 2 3 "Paravaccinia virus". Wikipedia. 2017-10-25.
- ↑ "http://viralzone.expasy.org/150?outline=all_by_species". viralzone.expasy.org. Retrieved 2017-11-04. External link in
|title=(help) - ↑ "ProMED-mail". www.promedmail.org. Retrieved 2017-11-04.
- ↑ Scagliarini, Alessandra; Vaccari, Francesca; Turrini, Filippo; Bianchi, Alessandro; Cordioli, Paolo; Lavazza, Antonio (April 2011). "Parapoxvirus Infections of Red Deer, Italy". Emerging Infectious Diseases. 17 (4): 684–687. doi:10.3201/eid1704.101454. ISSN 1080-6040. PMC 3377414. PMID 21470460.