Oxygen cycle
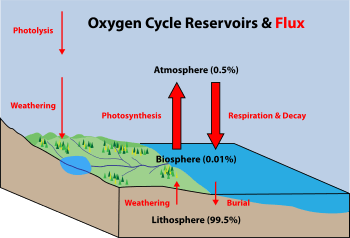
The oxygen cycle is the biogeochemical cycle of oxygen within its four main reservoirs: the atmosphere (air), the total content of biological matter within the biosphere (the global sum of all ecosystems), the hydrosphere (the combined mass of water found on, under, and over the surface of planet Earth), and the lithosphere/Earth's crust. The main driving factor of the oxygen cycle is photosynthesis, which is responsible for the modern Earth's atmosphere and life on Earth in its current form (see the Great Oxygenation Event).
Reservoirs

By far the largest reservoir of Earth's oxygen is within the silicate and oxide minerals of the crust and mantle (99.5% by weight). The Earth's atmosphere, hydrosphere and biosphere together weigh less than 0.05% of the Earth's total mass. Oxygen is one of the most abundant elements on Earth and represents a large portion of each main reservoir:
- Atmosphere is 20.9% oxygen by volume present mainly as free oxygen molecules (O2) with other oxygen-containing molecules including ozone (O3), carbon dioxide (CO2), water vapor (H2O), and sulfur and nitrogen oxides (SO2, NO, N2O, etc.)
- Biosphere is 22% oxygen by volume present mainly as a component of organic molecules (CxHxNxOx) and water molecules
- Hydrosphere is 33% oxygen by volume present mainly as a component of water molecules with dissolved molecules including free oxygen and carbonic acids (HxCO3)
- Lithosphere is 94% oxygen by volume present mainly as silica minerals (SiO2) and other oxide minerals
Movement of oxygen between the reservoirs is facilitated in large part by the presence of atmospheric free oxygen. The main source of atmospheric free oxygen is photosynthesis, which produces sugars and free oxygen from carbon dioxide and water:
Photosynthesizing organisms include the plant life of the land areas as well as the phytoplankton of the oceans. The tiny marine cyanobacterium Prochlorococcus was discovered in 1986 and accounts for more than half of the photosynthesis of the open ocean.[1]
An additional source of atmospheric free oxygen comes from photolysis, whereby high-energy ultraviolet radiation breaks down atmospheric water and nitrous oxide into component atoms. The free H and N atoms escape into space, leaving O2 in the atmosphere:
The main way free oxygen is lost from the atmosphere is via respiration and decay, mechanisms in which animal life and bacteria consume oxygen and release carbon dioxide.
The lithosphere also consumes atmospheric free oxygen by chemical weathering and surface reactions. An example of surface weathering chemistry is formation of iron oxides (rust):
Oxygen is also cycled between the biosphere and lithosphere. Marine organisms in the biosphere create calcium carbonate shell material (CaCO3) that is rich in oxygen. When the organism dies, its shell is deposited on the shallow sea floor and buried over time to create the limestone sedimentary rock of the lithosphere. Weathering processes initiated by organisms can also free oxygen from the lithosphere. Plants and animals extract nutrient minerals from rocks and release oxygen in the process.
Capacities and fluxes
The following tables offer estimates of oxygen cycle reservoir capacities and fluxes. These numbers are based primarily on estimates from (Walker, J. C. G.[2]):
Table 1: Major reservoirs involved in the oxygen cycle
| Reservoir | Capacity (kg O2) |
Flux in/out (kg O2 per year) |
Residence time (years) |
|---|---|---|---|
| Atmosphere | 1.4×1018 | 3×1014 | 4500 |
| Biosphere | 1.6×1016 | 3×1014 | 50 |
| Lithosphere | 2.9×1020 | 6×1011 | 500000000 |
Table 2: Annual gain and loss of atmospheric oxygen (Units of 1010 kg O2 per year)
| Photosynthesis (land) Photosynthesis (ocean) Photolysis of N2O Photolysis of H2O |
16,500 13,500 1.3 0.03 |
| Total gains | ~ 30,000 |
| Losses - respiration and decay | |
| Aerobic respiration Microbial oxidation Combustion of fossil fuel (anthropogenic) Photochemical oxidation Fixation of N2 by lightning Fixation of N2 by industry (anthropogenic) Oxidation of volcanic gases |
23,000 5,100 1,200 600 12 10 5 |
| Losses - weathering | |
| Chemical weathering Surface reaction of O3 |
50 12 |
| Total losses | ~ 30,000 |
Ozone
The presence of atmospheric oxygen has led to the formation of ozone (O3) and the ozone layer within the stratosphere:
The ozone layer is extremely important to modern life as it absorbs harmful ultraviolet radiation:
References
- Cloud, P. and Gibor, A. 1970, The oxygen cycle, Scientific American, September, S. 110-123
- Fasullo, J., Substitute Lectures for ATOC 3600: Principles of Climate, Lectures on the global oxygen cycle
, http://paos.colorado.edu/~fasullo/pjw_class/oxygencycle.html
- Morris, R.M., OXYSPHERE - A Beginners' Guide to the Biogeochemical Cycling of Atmospheric Oxygen, https://web.archive.org/web/20041103093231/http://seis.natsci.csulb.edu/rmorris/oxy/Oxy.htm