Iron cycle
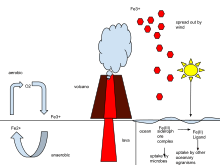
In ecology or geoscience, the iron cycle (Fe) is the biogeochemical cycle of iron through landforms, the atmosphere, and oceans. The iron cycle affects dust deposition and aerosol iron bioavailability.
Overview
Iron ranges in oxidation states from -2 to +7; however, in Earth's crust it is predominantly in its +2 (ferrous) or +3 (ferric) redox state. The cycling of iron between its ferrous and ferric oxidation states is referred to as the iron cycle. This process can be entirely abiotic, or facilitated by microorganisms. Some examples of this include the rusting of iron-bearing metals (in this case Fe2+ is abiotically oxidized to Fe3+) by oxygen, and the abiotic reduction of Fe3+ to Fe2+ by iron-sulfide minerals.[1] The iron cycle can also be facilitated by microorganisms, such as by Fe-oxidizing bacteria, which can oxidize Fe2+ to Fe3+, extracting one electron in the process for energy. Fe-reducing bacteria can reduce Fe3+ back to Fe2+ by utilizing it as a terminal electron acceptor.[2]
Iron is an important element on earth. It is 4th in abundance in the crust, and is an essential co-factor in many biological enzymes. Due to the high reactivity of Fe2+ with oxygen and low solubility of Fe3+, iron is a limiting nutrient to phytoplankton, the photosynthetic primary producers in the ocean. Thus, the iron cycle is intrinsically linked to the cycling of other biologically-important elements.
History of Iron Cycling on Earth
In the early earth, when atmospheric oxygen levels were 0.001% of those present today, dissolved iron was thought to have been a lot more abundant in the oceans, and thus more bioavailable to microbial life present in that era. At this time, before the onset of oxygenic photosynthesis, primary production may have been dominated by photoferrotrophs, which would obtain energy from sunlight, and use the electrons from Fe2+ to fix carbon.[citation needed]
References
- ↑ Ionescu, Danny; Heim, Christine; Polerecky, Lubos; Thiel, Volker; Beer, Dirk de (2015-03-16). "Biotic and abiotic oxidation and reduction of iron at circumneutral pH are inseparable processes under natural conditions". Geomicrobiology Journal. 32 (3–4): 221–230. doi:10.1080/01490451.2014.887393. ISSN 0149-0451.
- ↑ Shi, Liang; Dong, Hailiang; Reguera, Gemma; Beyenal, Haluk; Lu, Anhuai; Liu, Juan; Yu, Han-Qing; Fredrickson, James K. (10 2016). "Extracellular electron transfer mechanisms between microorganisms and minerals". Nature Reviews. Microbiology. 14 (10): 651–662. doi:10.1038/nrmicro.2016.93. ISSN 1740-1534. PMID 27573579. Check date values in:
|date=(help)
- Jickells, T. D., et al. (2005, April 1). Global iron connections between desert dust, ocean biogeochemistry, and climate. In Science, 308, 67 – 71.
External links