Monolaurin
 | |
| Names | |
|---|---|
| IUPAC name
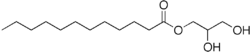
2,3-Dihydroxypropyl dodecanoate | |
| Other names
Glyceryl laurate; Monolauroylglycerin; Glycerol monolaurate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.043.929 |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C15H30O4 | |
| Molar mass | 274.40 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Monolaurin, also known as glycerol monolaurate, glyceryl laurate or 1-lauroyl-glycerol, is a monoglyceride. It is the mono-ester formed from glycerol and lauric acid. Its chemical formula is C15H30O4.
Uses
Monolaurin is most commonly used as a surfactant in cosmetics, such as deodorants. As a food additive it is also used as an emulsifier or preservative. Monolaurin is also taken as a dietary supplement.
Occurrence
Monolaurin is found in coconut oil and may be similar to other monoglycerides found in human breast milk.[1]
Lauric acid can be ingested in coconut oil and the human body converts it into monolaurin, but researchers are unsure of the conversion rates.[2] Because of this, it’s unknown how much coconut oil one would need to ingest to receive a therapeutic dose of monolaurin, however some articles suggest it may be upwards of 100-300mL/day making ingesting coconut oil unrealistic compared to monolaurin capsules.[3]
Pharmacology

Monolaurin has antibacterial, antiviral, and other antimicrobial effects in vitro,[4][5][6][7][8][9] but its clinical usefulness has not been established. Monolaurin is currently sold as a dietary supplement and is categorized in the United States by the Food and Drug Administration as generally recognized as safe (GRAS).[10]
Monolaurin is known to inactivate lipid-coated viruses by binding to the lipid-protein envelope of the virus, thereby preventing it from attaching and entering host cells, making infection and replication impossible.[11] Other studies show that Monolaurin disintegrates the protective viral envelope, killing the virus.[12][13] Monolaurin has been studied to inactivate many pathogens including Herpes simplex virus[14] and Chlamydia trachomatis.[15]
Monolaurin also shows promising effects against bacteria (both gram-positive and gram-negative), yeast, fungi, and protozoa. Bacteria including E. Coli,[16] yeast including Candida albicans,[17] Helicobacter pylori (H. pylori),[18] Giardia lamblia,[19] Staphylococcus aureus (Staph),[20] and other microbials have all been neutralized by monolaurin in scientific studies. Monolaurin also presented antibacterial and anti-biofilm properties against Borrelia burgdorferi and Borrelia garinii, the bacterium which cause Lyme Disease in humans.[21]
Furthermore, monolaurin does not seem to contribute to drug resistance,[22]
References
- ↑ Hegde, BM (2006). "View Point: Coconut Oil – Ideal Fat next only to Mother's Milk (Scanning Coconut's Horoscope)" (pdf). Journal of the Indian Academy of Clinical Medicine. 7: 16–19.
- ↑ "Monolaurin: Benefits, Dosage, and Side Effects". www.healthline.com. Retrieved 2017-12-02.
- ↑ "Pharmacological effects of coconut oil vs. monoglycerides" (PDF). inform Volume 16. June 2005.
- ↑ Li, Q; Estes, J. D.; Schlievert, P. M.; Duan, L; Brosnahan, A. J.; Southern, P. J.; Reilly, C. S.; Peterson, M. L.; Schultz-Darken, N; Brunner, K. G.; Nephew, K. R.; Pambuccian, S; Lifson, J. D.; Carlis, J. V.; Haase, A. T. (2009). "Glycerol monolaurate prevents mucosal SIV transmission". Nature. 458 (7241): 1034–8. doi:10.1038/nature07831. PMC 2785041. PMID 19262509.
- ↑ Preuss, H. G.; Echard, B.; Enig, M.; Brook, I.; Elliott, T. B. (2005). "Minimum inhibitory concentrations of herbal essential oils and monolaurin for gram-positive and gram-negative bacteria". Molecular and Cellular Biochemistry. 272 (1–2): 29–34. doi:10.1007/s11010-005-6604-1. PMID 16010969.
- ↑ Carpo, B. G.; Verallo-Rowell, V. M.; Kabara, J. (2007). "Novel antibacterial activity of monolaurin compared with conventional antibiotics against organisms from skin infections: an in vitro study". Journal of Drugs in Dermatology. 6 (10): 991–998. PMID 17966176.
- ↑ Isaacs, C. E. (2001). "The antimicrobial function of milk lipids". Advances in Nutritional Research. 10: 271–285. PMID 11795045.
- ↑ Lieberman, Shari; Enig, Mary G.; Preuss, Harry G. (2006). "A Review of Monolaurin and Lauric Acid:Natural Virucidal and Bactericidal Agents". Alternative and Complementary Therapies. 12 (6): 310–314. doi:10.1089/act.2006.12.310.
- ↑ Projan, S. J.; Brown-Skrobot, S.; Schlievert, P. M.; Vandenesch, F.; Novick, R. P. (1994). "Glycerol monolaurate inhibits the production of beta-lactamase, toxic shock toxin-1, and other staphylococcal exoproteins by interfering with signal transduction". Journal of Bacteriology. 176 (14): 4204–4209. PMC 205630. PMID 8021206.
- ↑ "CFR - Code of Federal Regulations Title 21". www.accessdata.fda.gov.
- ↑ Isaacs, CE; Kim, KS; Thormar, H (6 June 1994). "Inactivation of enveloped viruses in human bodily fluids by purified lipids". Annals of the New York Academy of Sciences. 724: 457–64. PMID 8030973.
- ↑ Thormar, H; Isaacs, C E; Brown, H R; Barshatzky, M R; Pessolano, T (1 January 1987). "Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides". Antimicrobial Agents and Chemotherapy. 31 (1): 27–31. doi:10.1128/aac.31.1.27. PMC 174645. PMID 3032090.
- ↑ Arora, R; Chawla, R; Marwah, R; Arora, P; Sharma, RK; Kaushik, V; Goel, R; Kaur, A; Silambarasan, M; Tripathi, RP; Bhardwaj, JR (2011). "Potential of Complementary and Alternative Medicine in Preventive Management of Novel H1N1 Flu (Swine Flu) Pandemic: Thwarting Potential Disasters in the Bud". Evidence-based Complementary and Alternative Medicine. 2011: 586506. doi:10.1155/2011/586506. PMC 2957173. PMID 20976081.
- ↑ Sands, J; Auperin, D; Snipes, W (January 1979). "Extreme sensitivity of enveloped viruses, including herpes simplex, to long-chain unsaturated monoglycerides and alcohols". Antimicrobial Agents and Chemotherapy. 15 (1): 67–73. doi:10.1128/aac.15.1.67. PMID 218499.
- ↑ Bergsson, G; Arnfinnsson, J; Karlsson, SM; Steingrímsson, O; Thormar, H (September 1998). "In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides". Antimicrobial Agents and Chemotherapy. 42 (9): 2290–4. PMID 9736551.
- ↑ Bergsson, G; Arnfinnsson, J; Steingrímsson, O; Thormar, H (November 2001). "In vitro killing of Candida albicans by fatty acids and monoglycerides". Antimicrobial Agents and Chemotherapy. 45 (11): 3209–12. doi:10.1128/AAC.45.11.3209-3212.2001. PMID 11600381.
- ↑ Bergsson, G; Arnfinnsson, J; Steingrímsson, O; Thormar, H (November 2001). "In vitro killing of Candida albicans by fatty acids and monoglycerides". Antimicrobial Agents and Chemotherapy. 45 (11): 3209–12. doi:10.1128/AAC.45.11.3209-3212.2001. ISSN 0066-4804. PMC 90807. PMID 11600381.
- ↑ Petschow, BW; Batema, RP; Ford, LL (February 1996). "Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids". Antimicrobial Agents and Chemotherapy. 40 (2): 302–6. PMID 8834870.
- ↑ Zeinab, Hassanein Fahmy; Eman, Aly; Ibrahim, shalsh; Amira, H. Mohamed (29 January 2014). "The effect of medium chain saturated fatty acid (monolaurin) on levels of the cytokines on experimental animal in Entamoeba histolytica and Giardia lamblia infection". African Journal of Pharmacy and Pharmacology. 8 (4): 106–114. doi:10.5897/AJPP2013.3839.
- ↑ Ruzin, A; Novick, RP (May 2000). "Equivalence of lauric acid and glycerol monolaurate as inhibitors of signal transduction in Staphylococcus aureus". Journal of Bacteriology. 182 (9): 2668–71. PMID 10762277.
- ↑ Goc, A; Niedzwiecki, A; Rath, M (December 2015). "In vitro evaluation of antibacterial activity of phytochemicals and micronutrients against Borrelia burgdorferi and Borrelia garinii". Journal of Applied Microbiology. 119 (6): 1561–72. doi:10.1111/jam.12970. PMID 26457476.
- ↑ Carpo, BG; Verallo-Rowell, VM; Kabara, J (October 2007). "Novel antibacterial activity of monolaurin compared with conventional antibiotics against organisms from skin infections: an in vitro study". Journal of Drugs in Dermatology. 6 (10): 991–8. ISSN 1545-9616. PMID 17966176.