MitraClip
| MitraClip | |
|---|---|
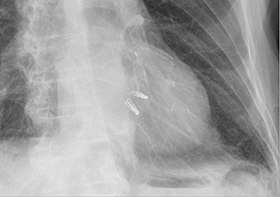 Chest radiograph showing two MitraClips projecting over the heart. |
MitraClip (mitral clip) is a medical device used to treat mitral valve regurgitation for individuals who should not have open-heart surgery. It is implanted via a transcatheter technique and involves suturing together the anterior and posterior mitral valve leaflets.[1]
Medical use
MitraClip is used for patients with severe mitral valve regurgitation that is refractory to medical therapy. Open-heart surgery remains the preferred treatment option when possible, due to the effectiveness and long-term record of the procedure in reducing mitral valve regurgitation.[1] Certain causes of mitral regurgitation, notably rheumatic fever, can be considered contraindications to having the procedure.
Adverse effects and complications
The most common complication of transcatheter mitral valve repair is access site bleeding, although transfusion is generally required less often than with surgical mitral valve repair. Rare but serious complications can include infective endocarditis, mitral stenosis, and device embolization. In general, major adverse events in a 30-day post-procedural time period are significantly lower with a transcatheter versus surgical approach.
History
MitraClip was developed by Evalve Inc., which became a division of Abbott Laboratories.
MitraClip was first implanted in 2003, obtained the CE mark Europe in 2008, and in 2013 was approved by the FDA.[2] The FDA obligated the company to run post-marketing studies to confirm the safety and efficacy of the device.[3]
Abbott expanded its MitraClip training process in 2016, after several instances of surgical complications.[4]
This medical device company also funded the researchers’ project, the Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients with Functional Mitral Regurgitation (COAPT) initiative.[5]
References
- 1 2 Wan B, Rahnavardi M, Tian DH, Phan K, Munkholm-Larsen S, Bannon PG, Yan TD (November 2013). "A meta-analysis of MitraClip system versus surgery for treatment to severe mitral regurgitation". Ann Cardiothorac Surg. 2 (6): 683–692. doi:10.3978/j.issn.2225-319X.2013.11.02. PMC 3857006. PMID 24349969.
- ↑ Feldman T (March 2014). "Rollout of the MitraClip". Latest in Cardiology. American College of Cardiology.
- ↑ "Approval letter for P100009" (PDF). FDA. October 24, 2013.
- ↑ Rubenfire A, Rice S (March 2016). "MitraClip recall shines spotlight on surgeon training, testing concerns". Modern Healthcare.
- ↑ O'Donnel D., Tiny New Implant ‘MitraClip’ May Usher Revolution In The Treatment Of Heart Failure, Evolving-Science