Methenium
3).
In organic chemistry, methenium (also called methylium, the methyl cation, or protonated methylene) is a positive ion with the formula CH+
3. It can be viewed as a methylene radical (:CH
2) with an added proton (H+
), or as a methyl radical (•CH
3) with one electron removed. It is a carbocation and an enium ion, making it the simplest of the carbenium ions.[1]
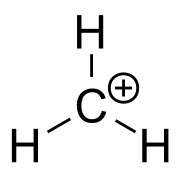
Structure
The methenium ion is planar or nearly planar, with threefold symmetry.[1]
Preparation and reactions
For mass spectrometry studies at low pressure, methenium can be obtained by ultraviolet photoionization of methyl radical,[1] or by collisions of monatomic cations like C+
and Kr+
with neutral methane.[2] In such conditions, it will react with acetonitrile CH
3CN to form the ion (CH
3)
2CN+
.[3]
Upon capture of a low-energy electron (less than 1 eV), it will spontaneously dissociate.[4]
It is seldom encountered as an intermediate in the condensed phase. It is proposed as a reactive intermediate that forms upon protonation or hydride abstraction of methane with FSO3H-SbF5. The methenium ion is very reactive, even towards alkanes.[5]
References
- 1 2 3 L. Golob, N. Jonathan, A. Morris, M. Okuda, K.J. Ross (1972), "The first ionization potential of the methyl radical as determined by photoelectron spectroscopy". Journal of Electron Spectroscopy and Related Phenomena, volume 1, issue 5, pp. 506–508 doi:10.1016/0368-2048(72)80022-7
- ↑ R.B. Sharma , N.M. Semo , W.S. Koski (1987), "Dynamics of the reactions of methylium, methylene radical cation, and methyliumylidene with acetylene". Journal of Physical Chemistry, volume 91, issue 15, pp. 4127–4131 doi:10.1021/j100299a037
- ↑ Murray J. McEwan, Arthur B. Denison, Wesley T. Huntress Jr., Vincent G. Anicich, J. Snodgrass, M.T. Bowers (1989), "Association reactions at low pressure. 2. The methylium/methyl cyanide system". Journal of Physical Chemistry, volume 93, issue 10, pp. 4064–4068. doi:10.1021/j100347a039
- ↑
E.M. Bahati, M. Fogle, C.R. Vane, M.E. Bannister, R.D. Thomas and V. Zhaunerchyk (2009),
"Electron-impact dissociation of CD+
3 and CH+
3 ions producing CD+
2, CH+
and C+
fragment ions". Physical Review, volume 79, article 052703 doi:10.1103/PhysRevA.79.052703 - ↑ Hogeveen, H.; Lukas, J.; Roobeek, C. F. (1969). "Trapping of the methyl cation by carbon monoxide; formation of acetic acid from methane". Journal of the Chemical Society D: Chemical Communications. 0 (16): 920. doi:10.1039/c29690000920. ISSN 0577-6171.