Mentha pulegium
| Mentha pulegium | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Plantae |
| Clade: | Angiosperms |
| Clade: | Eudicots |
| Clade: | Asterids |
| Order: | Lamiales |
| Family: | Lamiaceae |
| Genus: | Mentha |
| Species: | M. pulegium |
| Binomial name | |
| Mentha pulegium | |
Mentha pulegium, commonly (European) pennyroyal, or pennyrile, also called squaw mint, mosquito plant[1] and pudding grass,[2] is a species of flowering plant in the Lamiaceae family, or mint family, native to Europe, North Africa, and the Middle East.[3] Crushed pennyroyal leaves emits a very strong fragrance similar to spearmint. Pennyroyal is a traditional folk remedy, emmenagogue, abortifacient, and culinary herb. European pennyroyal is related to an American species, Hedeoma pulegioides. Though they differ in genera, they share similar chemical properties.[4]
History
Ancient to modern use
Documented use of pennyroyal dates back to ancient Greek, Roman, and Medieval cultures. Its name – although of uncertain etymology – is associated with Latin pulex (flea), alluding to the manner it was used to drive away fleas when smeared on the body.[5] Pennyroyal was commonly incorporated as a cooking herb by the Greeks and Romans. A large number of the recipes in the Roman cookbook of Apicius called for the use of pennyroyal, often along with such herbs as lovage, oregano and coriander. Although it was commonly used for cooking also in the Middle Ages, it gradually fell out of use as a culinary herb and is seldom used as such today.[6]
Records from Greek and Roman physicians and scholars contain information pertaining to pennyroyal's medicinal properties, as well as recipes used to prepared it. Pliny the Elder, in his encyclopedia Naturalis Historia (Natural History), described the plant as an emmenagogue, and that it also expelled a dead fetus.[7] Galen only listed pennyroyal as an emmenagogue, as did Oribasius. Roman and Greek writers Quintus Serenus Sammonicus and Aspasia however both agreed that pennyroyal, when served in tepid water, was an effective abortive method.[7] A medical text on gynecology attributed to Cleopatra (though it was never written by her but by a female Greek physician Metrodora) recommends the use of pennyroyal with wine to induce abortions.[7]
In regard to its contraceptive properties, it was referred to in a joking manner in Aristophanes' play Peace (421 BCE). The god Hermes provides the male character Trygaios a female companion; when Trygaios asks if there would be a problem if she became pregnant, Hermes responds, "Not if you add a dose of pennyroyal."[5] In a similar manner, in Aristophanes' comedy Lysistrata, after a pregnant female character on stage is told to withhold her body sexually from her husband, a slender female character, in comparison to the pregnant woman, is described as "a very lovely land Well croppy, and trimmed and spruced with pennyroyal."[5]
Early settlers in colonial Virginia used dried pennyroyal to eradicate pests. Pennyroyal was such a popular herb that the Royal Society published an article on its use against rattlesnakes in the first volume of its Philosophical Transactions in 1665.[8] 17th century apothecary and physician Nicholas Culpeper mentioned pennyroyal in his medical text The English Physitian, published in 1652.[9] In addition to its abortive properties, Culpeper recommends its use for other gastrointestinal ailments, such as constipation, hemorrhoids, as well as itching and blemishes to the skin, even toothaches.[9]
Pennyroyal continued to be used up through the 20th and 21st centuries. Its oil is still commercially available today, though little is known about the appropriate dosages for humans. Scientists therefore likely consider it unsafe for use, as it is potentially toxic.[10]
Attributed contributions to death
The following are reported cases in which pennyroyal toxicity resulted in death:
- 1897 – A 23-year-old British woman died eight days after swallowing a tablespoon of pennyroyal in order to induce menstruation.[11]
- Circa 1909 – The Supreme Court of Indiana convicted a Mr. Carter of prescribing and administering pennyroyal pills to a pregnant woman who died two months after her miscarriage.[12]
- August 1912 – A 16-year-old girl from Maryland consumed 36 pennyroyal pills to induce abortion. An autopsy revealed that the herbal abortion was only partially successful.[11]
- November 1978 – An 18-year-old pregnant woman from Denver, Colorado, died after ingesting one ounce of concentrated pennyroyal oil in an effort to abort her fetus. Prior to her death, the victim had reported using the dried leaves of the plant to induce menstruation with no ill effects.[13]
- July 1994 – Kris Humphrey, a 24-year-old woman, died in California.[14] At the time of her death, Humphrey unknowingly had an ectopic pregnancy, and drank an herbal tea made with pennyroyal extract. According to one news report, there is disagreement over whether Humphrey died as a result of the ectopic pregnancy or from pennyroyal poisoning.[14]
Uses
_-_Mentha_pulegium_-_Pennyroyal_(Squaw_mint%2C_Mosquito_plant%2C_Pudding_grass)_-_%D0%91%D0%BB%D0%B0%D1%82%D0%BD%D0%B0_%D0%BC%D0%B5%D0%BD%D1%82%D0%B0_-_Polei-Minze_(28506613866).jpg)
Pennyroyal is frequently used as an insecticide and pest repellant.[15] As a pest repellant, it is used to keep fleas away from household animals as well as on humans to ward off gnats and mosquitos. Some flea collars for pets have pennyroyal oil or the herb can be crushed in the lining. Humans have also put crushed pennyroyal stems in their pockets or on their clothing to ward off unwanted insects.[16] However, when using the pennyroyal plant as a pest repellant, the use of the concentrated pennyroyal oil should be avoided. Pennyroyal oil can be extremely toxic to both humans and animals, even in small quantities. With the use of pennyroyal around animals and humans comes the risk of it being absorbed through the skin and causing negative health effects. The less concentrated leaves of the plant should be used instead as an insect repellant.
Pennyroyal has historically also been used as a mint flavoring in herbal teas and foods. Pennyroyal tea has been used for cold relief, fevers, coughs, indigestion, liver and kidney problems and headaches.[17] The fresh or dried leaves of pennyroyal have also been used when treating influenza, abdominal cramps, to induce sweating, as well as in the treatment of diseases such as smallpox and tuberculosis.[18] To make the tea, the leaves of the plant are boiled in hot water. The lower concentration of toxic chemicals in these teas are less harmful than pennyroyal oil. It is recommended that people only drink pennyroyal tea periodically, as it is taxing on the body and should not be drunk on a regular basis. Consumption of pennyroyal tea can be fatal to infants and children.[19]
The pennyroyal plant has also been used as an emmenagogue or perhaps most famously as an abortifacient.[20] Chemicals in the pennyroyal plant cause the uterine lining to contract, causing a woman's uterine lining to shed. Women who struggle with regulating their menstrual cycle or suffer from a cystic ovary syndrome may choose to drink pennyroyal tea. Pennyroyal tea is subtle enough to induce menstrual flow with minimal risk of negative health effects. More concentrated versions of the plant, such as the oil, are much more toxic and will likely force a miscarriage if ingested by a pregnant woman.[21] Since the U.S. Congress passed the Dietary Supplement Health and Education Act in October 1994 all manufactured forms of pennyroyal in the United States have carried a warning label against its use by pregnant women, but pennyroyal is not regulated by the U.S. Food and Drug Administration.[22]
Toxicity

Pennyroyal is toxic to humans and has differing effects dependent on the volume and concentration ingested. The most concentrated and toxic form of the pennyroyal plant is pennyroyal oil. The oil contains 80% to 92% of cyclohexanone pulegone. Pulegone, the molecule in highest concentration in the pennyroyal plant, causes a variety of ailments in those who ingest it and is what causes the plant to have its peppermint flavor.[23]
Symptoms that may persist after ingesting a small dose (<10 mL) of pennyroyal oil are nausea, vomiting, abdominal pain and dizziness. Larger volumes may result in multiorgan failure that could lead to death. There are no current toxicokinetics studies performed on humans for the effects of pulegone, but there are some studies performed on other mammals. When pulegone is ingested, it is broken down by the liver and reacts to form multiple toxic metabolites that can wreak havoc in the body. Some identified metabolites are menthofuran, piperitenone, piperitone, and menthone.[24]
Studies conducted on the toxicity of pulegone
- Study conducted on rats shows that pulegone can cause delayed action potential in cardiac muscles.[25]
- Study conducted shows pulegone damaged ovarian tissue in mice. Increased dosages correlated to more severe damage.[26]
- In a study, pulegone was given to female rats through oral administration in doses of 0, 75, or 150 mg/kg of body weight. Individual studies lasted for 4–6 weeks and increased instance of urothelial tumors appeared as a result.[24]
In the first study listed above, pulegone reportedly had relaxant effects on vascular smooth muscles, myometrium, the bladder, intestine and the trachea of rats. The relaxation effects on these organs can be attributed to pulegone blocking the ion channels for Ca2+ and K+ to enter and exit the cell, respectively. This hinders the ability of the cell to achieve its necessary action potential for a nerve impulse to fire, relaxing the muscle.
In the second study listed, the impact of pulegone on follicular growth in mice was tested. In the mice injected with pulegone, there was much lower follicular distribution per ovary than those not injected. Ovarian follicles secrete hormones that influence a woman's menstruation cycle. Oestrogen levels are lower than normal when follicle numbers are low and it is possible that this can induce abortion or prevent ovulation.
In the third study, carcinomas in the bladder occurred at higher rates among mice that were injected with pulegone. The mechanism of action that causes these effects involves cytotoxicity and regenerative cell proliferation.
Chemistry
Structure and reactivity

The active chemical in pennyroyal is pulegone. Pulegone is a ketone and on the cellular level, ketones can act as enzyme inhibitors. The carbonyl center of the pulegone structure acts as a strong electrophile, causing active sites on enzymes to bind with pulegone instead of the target protein.
The endocyclic double bond found in pulegone is vital to the activation and binding mechanism of the molecule and causes it to be an effective hepatotoxin. When ingested, pulegone targets the liver and kidney, among other organs. Studies have been conducted on rats show that one of the main effects is the inhibition of contractile activity in the myometrium and death by kidney failure. Also found in the study, long term exposure to pennyroyal increased incidences of urinary bladder tumors.[24]
Mechanism of action
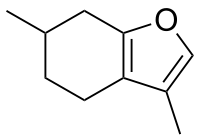
The exact mechanism of action by which pennyroyal induces menses and abortions in humans is still unknown. Studies using animal models speculate the source of liver toxicity is due to one of the many constituents the plant contains: pulegone, a monoterpene. Pulegone is metabolized by cytochrome P450 (CYP 1A2 and 2E1) and converted to several toxins.[27] Both in vitro and in vivo studies have found the pulegone metabolite menthofuran to be an inhibitor of CYP2A6, accounting for a significant degree of pennyroyal's hepatotoxicity.[28][29][30]
The exact pathway pulegone undergoes to be converted to menthofuran is unknown, but one study strongly suggested it included oxidation of an allylic methyl group (from CYP450), intramolecular cyclization to form a hemiketal, and subsequent dehydration to form the furan.[29] Additionally, pulegone and menthofuran may deplete glutathione levels, leaving hepatocytes vulnerable to free radical damage.[29]
Treatment
There is no known antidote for pennyroyal toxicity.[20] Case studies involving pennyroyal poisonings have reported the use of gastric lavages and administration of emetics, or vomiting inducing agents, like activated charcoal.[4] As glutathione depletion has been shown to regulate liver toxification, administration of N-acetylcysteine in similar doses as given for acetaminophen toxicity have been given to patients.[31]
A study testing pulegone toxicity found inhibitors of cytochrome P450, such as cobaltous chloride or piperonyl butoxide, blocked toxicity.[29] Such testing has not been expanded to humans, however, as the pennyroyal mechanism of toxicity is still not entirely understood.
See also
- Hedeoma pulegioides (American pennyroyal, a distantly related species)
References
- ↑ Gunby, Phil (1979). "Medical News: Plant Known for Centuries Still Causes Problems Today". Journal of the American Medical Association. 241 (21): 2246–2247. doi:10.1001/jama.241.21.2246.
- ↑ Keville, Kathi. (1994). Herbs: An Illustrated Encyclopedia. New York, New York: Friedman/Fairfax Publishers. Pp. 128.
- ↑ Kew World Checklist of Selected Plant Families
- 1 2 Ilene, Anderson B.; Walter, Mullen H.; James, Meeker E.; Siamak, Khojasteh-Bakht; Shimako, Oishi; Sidney, Nelson D.; Paul, Blanc D. (15 April 1996). "Pennyroyal Toxicity: Measurement of Toxic Metabolite Levels in Two Cases and Review of the Literature". Annals of Internal Medicine. 124 (8): 726–734. doi:10.7326/0003-4819-124-8-199604150-00004.
- 1 2 3 Riddle, John (April 1999). Eve's Herbs--A History of Contraception and Abortion in the West. Harvard University Press. ISBN 9780674270268.
- ↑ Kains, Maurice Grenville (1912). Culinary Herbs: Their Cultivation Harvesting Curing and Uses. New York: Orange Judd Company.
- 1 2 3 Riddle, John (January 1994). Contraception and Abortion from the Ancient World to the Renaissance. Harvard University Press. ISBN 9780674168763.
- ↑ "Of a Way of Killing Rattle-Snakes". Philosophical Transactions of the Royal Society of London. 1 (43). 1665. doi:10.1098/rstl.1665.0022.
- 1 2 Culpeper, Nicholas (1652). Culpeper's Enlglish Physician and Complete Herbal.
- ↑ "Pennyroyal". U.S. National Library of Medicine. Retrieved 27 April 2017.
- 1 2 Macht, David (1913). "THE ACTION OF SO-CALLED EMMENAGOGUE OILS ON THE ISOLATED UTERUS WITH A REPORT OF A CASE OF PENNYROYAL POISONING". Journal of the American Medical Association. 61 (2): 105–107. doi:10.1001/jama.1913.04350020031013.
- ↑ Editorial. (1909). "Medicolegal: Pyemia, "Pennyroyal Pills" and Evidence in Abortion Case. Journal of the American Medical Association LIII(11): 891.
- ↑ Sullivan, John B. Jr.; Rumack, Barry H.; Thomas, Harold Jr.; Peterson, Robert G.; Bryson, Peter (1978). "Pennyroyal Poisoning and Hepatotoxicity". Journal of the American Medical Association. 242 (26): 2873–2874. doi:10.1001/jama.242.26.2873.
- 1 2 Young, Gordon. (1995). "Lifestyle on Trial." Metro: Silicon Valley's Weekly Newspaper.
- ↑ Moore, Sarah; Ferreria Maia, Marta. "Plant-based insect repellents: a review of their efficacy, development and testing". NBIC. US National Library of Medicine National Institute of Health. Missing or empty
|url=(help) - ↑ Pleasant, Barbara. "What You Need to Know about Pennyroyal". Mother Earth News. Missing or empty
|url=(help) - ↑ "Pennyroyal Tea". Tea Infusion.
- ↑ "Pennyroyal". WebMD. Retrieved 27 April 2017.
- ↑ French, Larry. "Isolation of (R)-(+)-Pulegone from the European Pennyroyal Mint, Mentha Pulegium". The Chemical Educator. 5: 270–277. doi:10.1007/s00897020599a.
- 1 2 "Pulegone". Toxnet. National Library of Medicine. Retrieved 27 April 2017.
- ↑ "Herbal Abortifacients". Epigee Woman's Health.
- ↑ "Dietary Supplement Health and Education Act of 1994". National Institute of Health. 1994.
- ↑ Siano, F; Catalfamo, M; Cautela, D; Servillo, L; Castaldo, D (2005). "Analysis of pulegone and its enanthiomeric distribution in mint-flavoured food products". Food Additives & Contaminants. 22 (3): 197–203. doi:10.1080/02652030500041581.
- 1 2 3 Da Rocha, Mitscheli S.; Dodmane, Puttappa R.; Arnold, Lora L.; Pennington, Karen L.; Anwar, Muhammad M.; Adams, Bret R.; Taylor, Sean V.; Wermes, Clint; Adams, Tim B.; Cohen, Sam M. (12 April 2012). "Mode of Action of Pulegone on the Urinary Bladder of F344 Rats". Society of Toxicology. 128 (1). doi:10.1093/toxsci/kfs135.
- ↑ Santos-Miranda, Artur; Nei Gondim, Antonio; Menezes-Filho, Jose Evaldo Rodrigues; Lins Vasconcelos, Carla Marina; Cruz, Jader Santos; Roman-Campos, Danilo (September 15, 2014). "Pharmacological evaluation of R(+)-pulegone on cardiac excitability: role of potassium current blockahe and control of action potential waveform". Phytomedicine. 21 (10): 1146. doi:10.1016/j.phymed.2014.05.007.
- ↑ Souldouzi, R; Shalizar, A; Amani, S (June 2014). "Pulegone-induced damages in mice ovarian tissue correlates with CYP19 gene expression". Iranian Journal of Reproductive Medicine: 100–101.
- ↑ Cassileth, Barrie; Yeung, Simon; Gubili, Jyothirmai (2010). Herb-drug Interactions in Oncology. Shelton, CT: People's Medical Publishing House.
- ↑ Khojasteh-Bakh, S. C.; Koenigs, L. L.; Peter, R. M.; Trager, W. F.; Nelson, S. D. (July 1998). "(R)-(+)-Menthofuran is a potent, mechanism-based inactivator of human liver cytochrome P450 2A6". Drug Metabolism and Disposition. 26 (7): 701–704.
- 1 2 3 4 Gordon, W. P.; Huitric, A. C.; Seth, C. L.; McClanahan, R. H.; Nelson, S. D. (February 26, 1989). "The metabolism of the abortifacient terpene, (R)-(+)-pulegone, to a proximate toxin, menthofuran". Drug Metabolism and Disposition. 15 (5): 589–594.
- ↑ Thomassen, D.; Pearson, P. G.; Slattery, J. T.; Nelson, S. D. (January 17, 1991). "Partial characterization of biliary metabolites of pulegone by tandem mass spectrometry. Detection of glucuronide, glutathione, and glutathionyl glucuronide conjugates". Drug Metabolism and Disposition. 19 (5): 997–104.
- ↑ "Drug Record Pennyroyal Oil (Mentha Pulegium)". LiverTox. United States National Library of Medicine. Retrieved 2017-05-07.
External links
| Wikimedia Commons has media related to Mentha pulegium. |
![]()