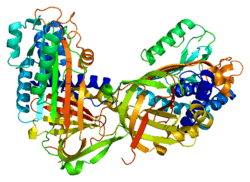Maspin
Maspin (mammary serine protease inhibitor) is a protein that in humans is encoded by the SERPINB5 gene.[5] This protein belongs to the serpin (serine protease inhibitor) superfamily.[5] SERPINB5 was originally reported to function as a tumor suppressor gene in epithelial cells, suppressing the ability of cancer cells to invade and metastasize to other tissues.[6] Furthermore, and consistent with an important biological function, Maspin knockout mice were reported to be non-viable, dying in early embryogenesis.[7] However, a subsequent study using viral transduction as a method of gene transfer (rather than single cell cloning) was not able to reproduce the original findings and found no role for maspin in tumour biology.[8] Furthermore, the latter study demonstrated that maspin knockout mice are viable and display no obvious phenotype.[8] These data are consistent with the observation that maspin is not expressed in early embryogenesis.[8] The precise molecular function of maspin is thus currently unknown.
Tissue distribution
Maspin is expressed in the skin, prostate, testis, intestine, tongue, lung, and the thymus.[5]
Serpin superfamily
Maspin is a member of the serpin superfamily of serine protease inhibitors.[5] The primary function of most members of this family is to regulate the breakdown of proteins by inhibiting the catalytic activity of proteinases. Through this mechanism of action, serpins regulate a number of cellular processes including phagocytosis, coagulation, and fibrinolysis.[9]
Serpins have a complex structure, a key component of which is the reactive site loop, RSL.[10] Inhibitory serpins transition between a stress and relaxed stage. The catalytic serine residue in the protease target attacks the stressed conformation of the RSL loop to form an acyl intermediate. The loop then undergoes a conformational change to the relaxed state irreversibly trapping the protease in an inactive state. Hence the serpin functions as a suicide inhibitor of the protease.[11] This transition does not occur in serpins that lack inhibitory activity.[10]
Function
Given its original reported role in cancer biology,[6] numerous studies have investigated a role for maspin in tumour metastasis.[12] However, to date no detailed molecular mechanism for maspin function in cell proliferation or tumour biology has been comprehensively described. Further, it is suggested that original reports of maspin as a tumor suppressor may reflect clonal artefacts rather than true maspin function.[8] Importantly, and in contrast to original reports, maspin knockout mice are viable, displaying no overt phenotype in the absence of suitable biological or environmental challenge.[8] Accordingly, the molecular function of maspin remains unclear.
From a structural perspective, maspin is a non-inhibitory and obligate intracellular member of the serpin superfamily.[13] Specifically, its RSL does not transition between a stressed and relaxed state following proteolytic cleavage.[14] This region is also shorter than the RSL loop in other serpins. Accordingly, in the absence of an obvious protease-related function, other targets of maspin have been suggested. For example, rather than being a protease inhibitor, maspin is proposed to function as an inhibitor of histone deacetylase 1 (HDAC1).[10][15]
Clinical significance
A comprehensive analysis of maspin expression in breast cancer revealed no significant correlation between maspin expression and overall survival, distant metastasis-free survival or recurrence-free survival.[8] Changes in maspin expression may, however, reflect the expression status of the known tumour suppressor PHLPP1.[8]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000206075 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000067006 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- 1 2 3 4 Khalkhali-Ellis Z (December 2006). "Maspin: the new frontier". Clin. Cancer Res. 12 (24): 7279–83. doi:10.1158/1078-0432.CCR-06-1589. PMC 3175762. PMID 17189399.
- 1 2 Zou Z, Anisowicz A, Hendrix MJ, Thor A, Neveu M, Sheng S, Rafidi K, Seftor E, Sager R (1994). "Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells". Science. 263 (5146): 526–9. doi:10.1126/science.8290962. PMID 8290962.
- ↑ Gao F, Shi H, Daughty C, Cella N, Zhang M (2004). "Maspin plays an essential role in early embryonic development". Development. 131 (7): 1479–89. doi:10.1242/dev.01048. PMID 14985257.
- 1 2 3 4 5 6 7 Teoh SS, Vieusseux J, Prakash M, Berkowicz S, Luu J, Bird CH, Law RH, Rosado C, Price JT, Whisstock JC, Bird PI (January 2014). "Maspin is not required for embryonic development or tumour suppression". Nat Commun. 5: 3164. doi:10.1038/ncomms4164. PMC 3905777. PMID 24445777.
- ↑ Potempa J, Korzus E, Travis J (June 1994). "The serpin superfamily of proteinase inhibitors: structure, function, and regulation". J. Biol. Chem. 269 (23): 15957–60. PMID 8206889.
- 1 2 3 Sager R, Sheng S, Pemberton P, Hendrix MJ (1996). "Maspin: a tumor suppressing serpin". Curr. Top. Microbiol. Immunol. 213 (1): 51–64. doi:10.1007/978-3-642-61107-0_4. PMID 8814994.
- ↑ Bailey CM, Khalkhali-Ellis Z, Seftor EA, Hendrix MJ (December 2006). "Biological functions of maspin". J. Cell. Physiol. 209 (3): 617–24. doi:10.1002/jcp.20782. PMID 17001697.
- ↑ Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. (2007). "Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin". Nature. 446 (7136): 690–4. doi:10.1038/nature05656. PMID 17377533.
- ↑ Teoh SS, Whisstock JC, Bird PI (2010). "Maspin (SERPINB5) is an obligate intracellular serpin". J Biol Chem. 285 (14): 10862–9. doi:10.1074/jbc.M109.073171. PMC 2856292. PMID 20123984.
- ↑ Pemberton PA, Wong DT, Gibson HL, Kiefer MC, Fitzpatrick PA, Sager R, et al. (1995). "The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin". J Biol Chem. 270 (26): 15832–7. doi:10.1074/jbc.270.26.15832. PMID 7797587.
- ↑ Lonardo F, Li X, Kaplun A, Soubani A, Sethi S, Gadgeel S, Sheng S (June 2010). "The natural tumor suppressor protein maspin and potential application in non small cell lung cancer". Curr. Pharm. Des. 16 (16): 1877–81. doi:10.2174/138161210791208974. PMC 2908495. PMID 20337574.
Further reading
- Sager R, Sheng S, Pemberton P, Hendrix MJ (1998). "Maspin. A tumor suppressing serpin". Adv. Exp. Med. Biol. 425: 77–88. doi:10.1007/978-1-4615-5391-5_8. PMID 9433491.
- Sheng S (2006). "The promise and challenge toward the clinical application of maspin in cancer". Front. Biosci. 9: 2733–45. doi:10.2741/1432. PMID 15353310.
- Lockett J, Yin S, Li X, et al. (2006). "Tumor suppressive maspin and epithelial homeostasis". J. Cell. Biochem. 97 (4): 651–60. doi:10.1002/jcb.20721. PMID 16329135.
- Chen EI, Yates JR (2006). "Maspin and tumor metastasis". IUBMB Life. 58 (1): 25–9. doi:10.1080/15216540500531721. PMID 16540429.
- Khalkhali-Ellis Z (2007). "Maspin: the new frontier". Clin. Cancer Res. 12 (24): 7279–83. doi:10.1158/1078-0432.CCR-06-1589. PMC 3175762. PMID 17189399.
- Schneider SS, Schick C, Fish KE, et al. (1995). "A serine proteinase inhibitor locus at 18q21.3 contains a tandem duplication of the human squamous cell carcinoma antigen gene". Proc. Natl. Acad. Sci. U.S.A. 92 (8): 3147–51. doi:10.1073/pnas.92.8.3147. PMC 42122. PMID 7724531.
- Pemberton PA, Wong DT, Gibson HL, et al. (1995). "The tumor suppressor maspin does not undergo the stressed to relaxed transition or inhibit trypsin-like serine proteases. Evidence that maspin is not a protease inhibitory serpin". J. Biol. Chem. 270 (26): 15832–7. doi:10.1074/jbc.270.26.15832. PMID 7797587.
- Zou Z, Anisowicz A, Hendrix MJ, et al. (1994). "Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells". Science. 263 (5146): 526–9. doi:10.1126/science.8290962. PMID 8290962.
- Pemberton PA, Tipton AR, Pavloff N, et al. (1998). "Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface". J. Histochem. Cytochem. 45 (12): 1697–706. doi:10.1177/002215549704501213. PMID 9389773.
- Xia W, Lau YK, Hu MC, et al. (2000). "High tumoral maspin expression is associated with improved survival of patients with oral squamous cell carcinoma". Oncogene. 19 (20): 2398–403. doi:10.1038/sj.onc.1203535. PMID 10828881.
- Biliran H, Sheng S (2002). "Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin". Cancer Res. 61 (24): 8676–82. PMID 11751384.
- Blacque OE, Worrall DM (2002). "Evidence for a direct interaction between the tumor suppressor serpin, maspin, and types I and III collagen". J. Biol. Chem. 277 (13): 10783–8. doi:10.1074/jbc.M110992200. PMID 11788595.
- Dokras A, Gardner LM, Kirschmann DA, et al. (2002). "The tumour suppressor gene maspin is differentially regulated in cytotrophoblasts during human placental development". Placenta. 23 (4): 274–80. doi:10.1053/plac.2001.0784. PMID 11969337.
- Jiang N, Meng Y, Zhang S, et al. (2002). "Maspin sensitizes breast carcinoma cells to induced apoptosis". Oncogene. 21 (26): 4089–98. doi:10.1038/sj.onc.1205507. PMID 12037665.
- Odero-Marah VA, Khalkhali-Ellis Z, Schneider GB, et al. (2002). "Tyrosine phosphorylation of maspin in normal mammary epithelia and breast cancer cells". Biochem. Biophys. Res. Commun. 295 (4): 800–5. doi:10.1016/S0006-291X(02)00764-7. PMID 12127964.
- Maass N, Biallek M, Rösel F, et al. (2002). "Hypermethylation and histone deacetylation lead to silencing of the maspin gene in human breast cancer". Biochem. Biophys. Res. Commun. 297 (1): 125–8. doi:10.1016/S0006-291X(02)02136-8. PMID 12220518.
- Sood AK, Fletcher MS, Gruman LM, et al. (2002). "The paradoxical expression of maspin in ovarian carcinoma". Clin. Cancer Res. 8 (9): 2924–32. PMID 12231537.
- Son HJ, Sohn TS, Song SY, et al. (2003). "Maspin expression in human gastric adenocarcinoma". Pathol. Int. 52 (8): 508–13. doi:10.1046/j.1440-1827.2002.01381.x. PMID 12366809.
- Bass R, Fernández AM, Ellis V (2003). "Maspin inhibits cell migration in the absence of protease inhibitory activity". J. Biol. Chem. 277 (49): 46845–8. doi:10.1074/jbc.C200532200. PMID 12384513.
External links
- The MEROPS online database for peptidases and their inhibitors: I04.980
- maspin at the US National Library of Medicine Medical Subject Headings (MeSH)