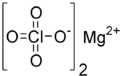
Magnesium perchlorate
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.030.086 |
PubChem CID |
|
| RTECS number | SC8925000 |
| UNII | |
| |
| |
| Properties | |
| Mg(ClO4)2 | |
| Molar mass | 223.206 g/mol |
| Appearance | white powder, deliquescent |
| Odor | odorless |
| Density | 2.21 g/cm3 (anhydrous) 1.98 g/cm3 (hexahydrate) |
| Melting point | 251 °C (484 °F; 524 K) (anhydrous) 95-100 °C (hexahydrate) |
| Boiling point | decomposition |
| 99.3 g/100 mL | |
| Solubility in ethanol | 23.96 g/100 mL |
| Hazards | |
| Safety data sheet | External MSDS |
| R-phrases (outdated) | R8, R36, R37, R38 |
| S-phrases (outdated) | S17, S26, S27, S36, S37, S39 |
| NFPA 704 | |
| Related compounds | |
Other cations |
Calcium perchlorate Barium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Magnesium perchlorate is a powerful oxidizing agent, with the formula Mg(ClO4)2. It is also a superior drying agent for gas analysis.
Magnesium perchlorate decomposes at 250 °C.[1] The heat of formation is -568.90 kJ mol−1.[2]
The enthalpy of solution is quite high, so reactions are done in large amounts of water to dilute it.
It is sold under the trade name anhydrone. Manufacture of this product on a semi-industrial scale was first performed by G. Frederick Smith in his garage in Urbana Illinois, but later at a permanent facility in Columbus, OH called G. Frederick Smith Chemical Co. He sold the magnesium perchlorate to A. H. Thomas Co., now Thomas Scientific, under the trade name Dehydrite.
It is used as desiccant to dry gas or air samples,[3][4] but is no longer advised, for use as a general desiccant, due to hazards inherent in perchlorates.[5] It is dried by heating at 220 °C under vacuum.
Magnesium perchlorate is created by the reaction of magnesium hydroxide and perchloric acid.
In 2011, a study conducted at the Georgia Institute of Technology revealed the presence of magnesium perchlorate on the planet Mars.[6] Being a drying agent, magnesium perchlorate retains water from the atmosphere and may release it when conditions are favorable and temperature is above 250K. Because briny solutions that contain magnesium perchlorate have a lower melting point than that of pure water, their abundance on Mars could serve as evidence that liquid water may exist on its surface, where temperature and pressure conditions would ordinarily cause water to freeze.
References
- ↑ CRC Handbook
- ↑ Lange's
- ↑ H. H. Willard, G. F. Smith (1922). "The Preparation and Properties of Magnesium Perchlorate and its Use as a Drying Agent". Journal of the American Chemical Society. 44 (10): 2255–2259. doi:10.1021/ja01431a022.
- ↑ L. Wu, H. He (1994). "Preparation of perlite-based magnesium perchlorate desiccant with colour indicator". The Chemical Educator. 41 (5): 633–637. doi:10.1016/0039-9140(94)80041-3.
- ↑ W. L. F. Armarego and C. Chai (2003). Purification of laboratory chemicals. Oxford: Butterworth-Heinemann. ISBN 0-7506-7571-3.
- ↑ https://www.letemps.ch/sciences/2015/09/28/eau-liquide-reperee-pentes-martiennes
Compounds containing perchlorate group | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HClO4 | He | ||||||||||||||||||
| LiClO4 | Be(ClO4)2 | B(ClO 4)− 4 B(ClO4)3 |
ROClO3 | N(ClO4)3 NH4ClO4 NOClO4 |
O | FClO4 | Ne | ||||||||||||
| NaClO4 | Mg(ClO4)2 | Al(ClO4)3 | Si | P | S | ClO− 4 ClOClO3 Cl2O7 |
Ar | ||||||||||||
| KClO4 | Ca(ClO4)2 | Sc(ClO4)3 | Ti(ClO4)4 | VO(ClO4)3 VO2(ClO4) |
Cr(ClO4)3 | Mn(ClO4)2 | Fe(ClO4)3 | Co(ClO4)2, Co(ClO4)3 |
Ni(ClO4)2 | Cu(ClO4)2 | Zn(ClO4)2 | Ga(ClO4)3 | Ge | As | Se | Br | Kr | ||
| RbClO4 | Sr(ClO4)2 | Y(ClO4)3 | Zr(ClO4)4 | Nb(ClO5)4 | Mo | Tc | Ru | Rh(ClO4)3 | Pd(ClO4)2 | AgClO4 | Cd(ClO4)2 | In(ClO4)3 | Sn(ClO4)4 | Sb | TeO(ClO4)2 | I | Xe | ||
| CsClO4 | Ba(ClO4)2 | Hf(ClO4)4 | Ta(ClO5)5 | W | Re | Os | Ir | Pt | Au | Hg2(ClO4)2, Hg(ClO4)2 |
Tl(ClO4)3 | Pb(ClO4)2 | Bi(ClO4)3 | Po | At | Rn | |||
| FrClO4 | Ra | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||||
| La | Ce(ClO4)x | Pr | Nd | Pm | Sm(ClO4)3 | Eu(ClO4)3 | Gd(ClO4)3 | Tb(ClO4)3 | Dy(ClO4)3 | Ho(ClO4)3 | Er(ClO4)3 | Tm(ClO4)3 | Yb(ClO4)3 | Lu(ClO4)3 | |||||
| Ac | Th(ClO4)4 | Pa | UO2(ClO4)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||||
