Ketosis
| Ketosis | |
|---|---|
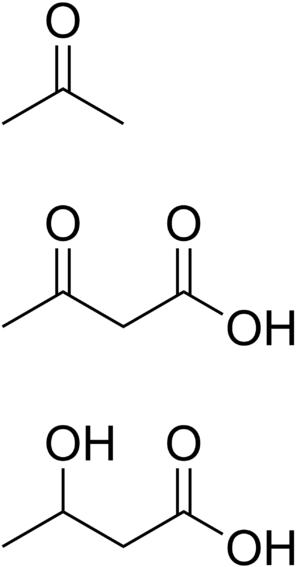 | |
| Ketone bodies: acetone, acetoacetic acid, and beta-hydroxybutyric acid | |
| Pronunciation |
|
| Specialty | Endocrinology specialty |
Ketosis is a metabolic state in which some of the body's energy supply comes from ketone bodies in the blood, in contrast to a state of glycolysis in which blood glucose provides energy. Generally, ketosis occurs when the body is metabolizing fat at a high rate and converting fatty acids into ketones.
Ketosis is a nutritional process characterised by serum concentrations of ketone bodies over 0.5 mM, with low and stable levels of insulin and blood glucose.[1][2] It is almost always generalized with hyperketonemia, that is, an elevated level of ketone bodies in the blood throughout the body. Ketone bodies are formed by ketogenesis when liver glycogen stores are depleted (or from metabolising medium-chain triglycerides[3]). Ketones can also be consumed in exogenous ketone foods and supplements.
The main ketone bodies used for energy are acetoacetate and β-hydroxybutyrate,[4] and the levels of ketone bodies are regulated mainly by insulin and glucagon.[5] Most cells in the body can use both glucose and ketone bodies for fuel, and during ketosis, free fatty acids and glucose synthesis (gluconeogenesis) fuel the remainder.
Longer-term ketosis may result from fasting or staying on a low-carbohydrate diet (ketogenic diet), and deliberately induced ketosis serves as a medical intervention for various conditions, such as intractable epilepsy, and the various types of diabetes.[6] In glycolysis, higher levels of insulin promote storage of body fat and block release of fat from adipose tissues, while in ketosis, fat reserves are readily released and consumed.[5][7] For this reason, ketosis is sometimes referred to as the body's "fat burning" mode.[8]
The difference between ketosis and ketoacidosis is the level of ketones in the blood. Ketosis is a physiological adaptation to a low carbohydrate environment like fasting or a ketogenic diet. There are situations (such as treatment-resistant epilepsy) where ketosis can be beneficial to health. Ketoacidosis is an acute life-threatening state requiring prompt medical intervention; its most common form is diabetic ketoacidosis where both glucose and ketone levels are significantly elevated.
Cause
Ketoacidosis
Ketone bodies are acidic, but acid-base homeostasis in the blood is normally maintained through bicarbonate buffering, respiratory compensation to vary the amount of CO2 in the bloodstream, hydrogen ion absorption by tissue proteins and bone, and renal compensation through increased excretion of dihydrogen phosphate and ammonium ions.[9] Prolonged excess of ketone bodies can overwhelm normal compensatory mechanisms, defined as acidosis if blood pH falls below 7.35.
There are two major causes of ketoacidosis:
- Most commonly, ketoacidosis is diabetic ketoacidosis (DKA), resulting from increased fat metabolism due to a shortage of insulin. It is associated primarily with type I diabetes, and may result in a diabetic coma if left untreated.[10]
- Alcoholic ketoacidosis (AKA) presents infrequently, but can occur with acute alcohol intoxication, most often following a binge in alcoholics with acute or chronic liver or pancreatic disorders. Alcoholic ketoacidosis occurs more frequently following methanol or ethylene glycol intoxication than following intoxication with uncontaminated ethanol.[11]
A mild acidosis may result from prolonged fasting or when following a ketogenic diet or a very low calorie diet.[12][13]
Diet
Ketosis is deliberately induced by use of a ketogenic diet as a medical intervention in cases of intractable epilepsy.[12] Other uses of low-carbohydrate diets remain controversial.[14][15] Carbohydrate deprivation to the point of ketosis has been argued to have both negative[16] and positive effects on health.[17][18] Ketosis can also be induced following periods of fasting (starvation),[19] and after consumption of ketogenic fats (such as medium chain triglycerides ) or exogenous ketones.[20]
Mechanism
The two sources of ketone bodies in the body are fatty acids in adipose tissue and ketogenic amino acids.[21][22] The main formation of ketone bodies is through ketogenesis.
Adipose tissue can be used to store fatty acids for regulating temperature and energy.[21] These fatty acids can be released by adipokine signaling of high glucagon and epinephrine levels, which inversely corresponds to low insulin levels. High glucagon and low insulin correspond to times of fasting or to times when blood glucose levels are low.[23] Fatty acids must be metabolized in mitochondria in order to produce energy, but free fatty acids cannot penetrate biological membranes due to their negative electrical charge. So coenzyme A is bound to the fatty acid to produce acyl-CoA, which is able to enter the mitochondria.
Once inside the mitochondrion, the dominant way that the bound fatty acids are used as fuel in cells is through β-oxidation, which cleaves two carbons off of the acyl-CoA molecule in every cycle to form acetyl-CoA.[24] Acetyl-CoA enters the citric acid cycle, where it undergoes an aldol condensation with oxaloacetate to form citric acid; citric acid then enters the tricarboxylic acid cycle (TCA), which harvests a very high energy yield per carbon in the original fatty acid.[25][26]
Acetyl-CoA can be metabolized through the TCA in any cell, but it can also undergo a different process in liver cells: ketogenesis, which produces ketone bodies.[27] Ketone bodies are also produced in mitochondria, and usually occur in response to low blood glucose levels.[28] When glucose levels are low, oxaloacetate is diverted away from the TCA cycle and is instead used to produce glucose de novo (gluconeogenesis). But when oxaloacetate is unavailable to condense with acetyl-CoA, acetyl-CoA cannot enter the cycle, and so the body has evolved an alternative way to harvest energy from it.
In ketogenesis, two acetyl-CoA molecules instead condense to form acetoacetyl-CoA via thiolase. Acetoacetyl-CoA momentarily combines with another acetyl-CoA via HMG-CoA synthase to form hydroxy-β-methylglutaryl-CoA. Hydroxy-β-methylglutaryl-CoA form the ketone body acetoacetate via HMG-CoA lyase. Acetoacetate can then reversibly convert to another ketone body—D-β-hydroxybutyrate—via D-β-hydroxybutyrate dehydrogenase. Alternatively, acetoacetate can spontaneously degrade to a third ketone body (acetone) and carbon dioxide, although the process generates much greater concentrations of acetoacetate and D-β-hydroxybutyrate. When blood glucose levels are low, ketone bodies can be exported from the liver to supply crucial energy to the brain.[28]
Along with the fatty acids, deaminated ketogenic amino acids can also be converted into intermediates in the citric acid cycle and produce ketone bodies.[22]
Diagnosis
Whether ketosis is taking place can be checked by using special urine test strips such as Ketostix. The strips have a small pad on the end, which the user dips in a fresh urine specimen. Within seconds, the strip changes color to indicate the level of acetoacetate ketone bodies, which reflects the degree of ketonuria, which, in turn, gives a rough estimate of the level of hyperketonemia in the body (see table below). Alternatively, some products targeted to diabetics such as the Abbott Precision Xtra or the Nova Max can be used to take a blood sample and measure the β-hydroxybutyrate ketone levels directly. Normal serum reference ranges for ketone bodies are 0.5–3.0 mg/dL, equivalent to 0.05–0.29 mmol/L.[29]
Also, when the body is in ketosis, one's breath may smell of acetone. This is due to the breakdown of acetoacetic acid into acetone and carbon dioxide exhaled through the lungs. Acetone is the chemical responsible for the smell of nail polish remover and some paint thinners.
| Urine value | Designation | Approximate serum concentration | |
|---|---|---|---|
| mg/dL | mmol/l | ||
| 0 | Negative | Reference range: 0.5–3.0[29] | 0.05–0.29[29] |
| 1+ | 5 (interquartile range (IQR): 1–9)[30] | 0.5 (IQR: 0.1–0.9)[31] | |
| 2+ | Ketonuria[32] | 7 (IQR: 2–19)[30] | 0.7 (IQR: 0.2–1.8)[31] |
| 3+ | 30 (IQR: 14–54)[30] | 3 (IQR: 1.4–5.2)[31] | |
| 4+ | Severe ketonuria[33] | – | – |
Severity
The concentration of ketone bodies may vary depending on diet, exercise, degree of metabolic adaptation and genetic factors. Ketosis can be induced when a ketogenic diet is followed for more than 3 days.[34] This induced ketosis is sometimes called nutritional ketosis.[35] This table shows the concentrations typically seen under different conditions[1]
| blood concentration (millimolar) | Condition |
|---|---|
| < 0.2 | not in ketosis |
| 0.2 - 0.5 | slight/mild ketosis |
| 0.5 - 3.0 | induced/nutritional ketosis |
| 2.5 - 3.5 | post-exercise ketosis |
| 3.0 - 6.0 | starvation ketosis |
| 15 - 25 | ketoacidosis |
Note that urine measurements may not reflect blood concentrations. Urine concentrations are lower with greater hydration, and after adaptation to a ketogenic diet the amount lost in the urine may drop while the metabolism remains ketotic. Most urine strips only measure acetoacetate, while when ketosis is more severe the predominant ketone body is β-hydroxybutyrate.[36] Unlike glucose, ketones are excreted into urine at any blood level. Ketoacidosis is a metabolic derangement that cannot occur in a healthy individual who can produce insulin, and should not be confused with physiologic ketosis.
Controversy
Some clinicians[37] regard eliminating carbohydrates as unhealthy and dangerous.[38] However, it is not necessary to eliminate carbohydrates from the diet completely to achieve ketosis. Other clinicians regard ketosis as a safe biochemical process that occurs during the fat-burning state.[35] Ketosis, which is accompanied by gluconeogenesis (the creation of glucose de novo from pyruvate), is the specific state that concerns some clinicians. However, it is unlikely for a normally functioning person to reach life-threatening levels of ketosis, defined as serum beta-hydroxybutyrate (B-OHB) levels above 15 millimolar (mM) compared to ketogenic diets among non diabetics, which "rarely run serum B-OHB levels above 3 mM."[39] This is avoided with proper basal secretion of pancreatic insulin. People who are unable to secrete basal insulin, such as type 1 diabetics and long-term type II diabetics, are liable to enter an unsafe level of ketosis, eventually resulting in a coma that requires emergency medical treatment. The anti-ketosis conclusions have been challenged by a number of doctors and advocates of low-carbohydrate diets, who dispute assertions that the body has a preference for glucose and that there are dangers associated with ketosis.[40][41]
Inuit people
The Inuit are often cited as an example of a culture that has lived for hundreds of years on a low-carbohydrate diet.[42] However, in multiple studies the traditional Inuit diet has not been shown to be a ketogenic diet.[43][44][45][46] Not only have multiple researchers been unable to detect any evidence of ketosis resulting from the traditional Inuit diet, but the ratios of fatty-acid to glucose were observed at well below the generally accepted level of ketogenesis.[44][47][45][46] Furthermore, studies investigating the fat yields from fully dressed wild ungulates, and the dietary habits of the cultures who rely on them, suggest that they are too lean to support a ketogenic diet.[48][49] With limited access to fat and carbohydrates, cultures such as the Nunamiut Eskimos—who relied heavily on caribou for subsistence—annually traded for fat and seaweed with coastal-dwelling Taremiut.[48]
Some Inuit consume as much as 15–20% of their calories from carbohydrates, largely from the glycogen found in raw meats.[43][44][47][45][50] Furthermore, the blubber, organs, muscle and skin of the diving marine mammals that the Inuit eat have significant glycogen stores that are able to delay postmortem degradation, particularly in cold weather.[51][52][53][54][55][56]
Moreover, recent studies show that the Inuit have evolved a number of rare genetic adaptations that make them especially well suited to eat large amounts of omega-3 fat.[57][58][59] And earlier studies showed that the Inuit have a very high frequency—68% to 81% in certain arctic coastal populations—of an extremely rare autosomal recessive mutation of the CPT1A gene—a key regulator of mitochondrial long-chain fatty-acid oxidation[60][61]—which results in a rare metabolic disorder known as carnitine palmitoyltransferase 1A (CPT1A) deficiency and promotes hypoketotic hypoglycemia—low levels of ketones and low blood sugar.[62] The condition presents symptoms of a fatty acid and ketogenesis disorder.[62] However, it appears highly beneficial to the Inuit[60] as it shunts free fatty acids away from liver cells to brown fat, for thermogenesis.[63][64] Thus the mutation may help the Inuit stay warm by preferentially burning fatty acids for heat in brown fat cells.[64] In addition to promoting low ketone levels, this disorder also typically results in hepatic encephalopathy (enlarged liver) and high infant mortality.[65] Inuit have been observed to have enlarged livers with an increased capacity for gluconeogenesis, and have greater capacity for excreting urea to remove ammonia, a toxic byproduct of protein breakdown.[57][66][67][68] Ethnographic texts have documented the Inuit's customary habit of snacking frequently [69] and this may well be a direct consequence of their high prevalence of the CPT1A mutation[70] as fasting, even for several hours, can be deleterious for individuals with that allele, particularly during strenuous exercise.[57][70] The high frequency of the CPT1A mutation in the Inuit therefore suggests that it is an important adaptation to their low carbohydrate diet and their extreme environment.[57][60][70]
In addition to the seaweed and glycogen carbohydrates mentioned above, the Inuit can access many plant sources. The stomach contents of caribou contain a large quantity of partially digested lichens and plants, which the Inuit once considered a delicacy. They also harvested reindeer moss and other lichens directly. The extended daylight of the arctic summer led to a profusion of plant life, and they harvested plant parts including berries, roots and stems, as well as mushrooms. They preserved some gathered plant life to eat during winter, often by dipping it in seal fat.[71]
Adaptation
While it is believed that carbohydrate intake after exercise is the most effective way of replacing depleted glycogen stores,[72][73] studies have shown that, after a period of 2–4 weeks of adaptation, physical endurance (as opposed to physical intensity) is unaffected by ketosis, as long as the diet contains high amounts of fat, relative to carbohydrates.[74] Some clinicians refer to this period of keto-adaptation as the "Schwatka imperative" after Frederick Schwatka, the explorer who first identified the transition period from glucose-adaptation to keto-adaptation.[75]
Veterinary medicine
In dairy cattle, ketosis is a common ailment that usually occurs during the first weeks after giving birth to a calf. Ketosis is in these cases sometimes referred to as acetonemia. A study from 2011 revealed that whether ketosis is developed or not depends on the lipids a cow uses to create butterfat. Animals prone to ketosis mobilize fatty acids from adipose tissue, while robust animals create fatty acids from blood phosphatidylcholine (lecithin). Healthy animals can be recognized by high levels of milk glycerophosphocholine and low levels of milk phosphocholine.[76] Point of care diagnostic tests are available and are reasonably useful.[77]
In sheep, ketosis, evidenced by hyperketonemia with beta-hydroxybutyrate in blood over 0.7 mmol/L, occurs in pregnancy toxemia.[78][79] This may develop in late pregnancy in ewes bearing multiple fetuses,[78][79] and is associated with the considerable glucose demands of the conceptuses.[80][81] In ruminants, because most glucose in the digestive tract is metabolized by rumen organisms, glucose must be supplied by gluconeogenesis,[82] for which propionate (produced by rumen bacteria and absorbed across the rumen wall) is normally the principal substrate in sheep, with other gluconeogenic substrates increasing in importance when glucose demand is high or propionate is limited.[83][84] Pregnancy toxemia is most likely to occur in late pregnancy because most fetal growth (and hence most glucose demand) occurs in the final weeks of gestation; it may be triggered by insufficient feed energy intake (anorexia due to weather conditions, stress or other causes),[79] necessitating reliance on hydrolysis of stored triglyceride, with the glycerol moiety being used in gluconeogenesis and the fatty acid moieties being subject to oxidation, producing ketone bodies.[78] Among ewes with pregnancy toxemia, beta-hydroxybutyrate in blood tends to be higher in those that die than in survivors.[85] Prompt recovery may occur with natural parturition, Caesarean section or induced abortion. Prevention (through appropriate feeding and other management) is more effective than treatment of advanced stages of ovine ketosis.[86]
See also
- Bioenergetics
- Ketoacidosis
- Ketogenic diet
- Ketonuria
- Low-carbohydrate diet
- Fasting
- Ketogenesis
- Spontaneous human combustion, for which acetone produced by ketosis has been suggested as a cause.
- Very-low-calorie diet
- Gluconeogenesis
References
- 1 2 Volek & Phinney, page 91.
- ↑ thefreedictionary.com/ketosis citing:
- The American Heritage® Medical Dictionary Copyright © 2007
- Mosby's Medical Dictionary, 8th edition. © 2009
- Dorland's Medical Dictionary for Health Consumers. © 2007
- ↑ Bach A, Schirardin H, Weryha A, Bauer M (October 1977). "Ketogenic response to medium-chain triglyceride load in the rat". The Journal of Nutrition. 107 (10): 1863–70. PMID 903830.
- ↑ Champe PC, Harvey RA. Lippincott’s Illustrated Reviews: Biochemistry. Lippincott Williams & Wilkins.
- 1 2 Johnston DG, Pernet A, McCulloch A, Blesa-Malpica G, Burrin JM, Alberti KG (1982). "Some hormonal influences on glucose and ketone body metabolism in normal human subjects". Ciba Foundation Symposium. Novartis Foundation Symposia. 87: 168–91. doi:10.1002/9780470720691.ch10. ISBN 9780470720691. PMID 6122546.
- ↑ Kossoff EH, Freeman JM, Turner Z, Rubenstein JE (2011). Ketogenic Diets: Treatments for Epilepsy and Other Diseases. Demos Health.
- ↑ Manninen AH (December 2004). "Metabolic effects of the very-low-carbohydrate diets: misunderstood "villains" of human metabolism". Journal of the International Society of Sports Nutrition. 1 (2): 7–11. doi:10.1186/1550-2783-1-2-7. PMC 2129159. PMID 18500949.
- ↑ Paoli A, Rubini A, Volek JS, Grimaldi KA (August 2013). "Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets". European Journal of Clinical Nutrition. 67 (8): 789–96. doi:10.1038/ejcn.2013.116. PMC 3826507. PMID 23801097.
- ↑ Marshall WJ, Bangert SK (2008). Clinical biochemistry: metabolic and clinical aspects. Elsevier Health Sciences. pp. 67–80. ISBN 978-0-443-10186-1.
- ↑ Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA (December 2006). "Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association". Diabetes Care. 29 (12): 2739–48. doi:10.2337/dc06-9916. PMID 17130218.
- ↑ Kraut JA, Kurtz I (January 2008). "Toxic alcohol ingestions: clinical features, diagnosis, and management". Clinical Journal of the American Society of Nephrology. 3 (1): 208–25. doi:10.2215/CJN.03220807. PMID 18045860.
- 1 2 Hartman AL, Vining EP (January 2007). "Clinical aspects of the ketogenic diet". Epilepsia. 48 (1): 31–42. doi:10.1111/j.1528-1167.2007.00914.x. PMID 17241206.
- ↑ Delbridge E, Proietto J (2006). "State of the science: VLED (Very Low Energy Diet) for obesity". Asia Pacific Journal of Clinical Nutrition. 15 Suppl: 49–54. PMID 16928661.
- ↑ Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, Szapary PO, Rader DJ, Edman JS, Klein S (May 2003). "A randomized trial of a low-carbohydrate diet for obesity". The New England Journal of Medicine. 348 (21): 2082–90. doi:10.1056/NEJMoa022207. PMID 12761365.
- ↑ Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD, Bravata DM (April 2003). "Efficacy and safety of low-carbohydrate diets: a systematic review". JAMA. 289 (14): 1837–50. doi:10.1001/jama.289.14.1837. PMID 12684364.
- ↑ Plaskett, L. G. (September 2003). "On the Essentiality of Dietary Carbohydrate". Journal of Nutritional & Environmental Medicine. 13 (3): 161–168. doi:10.1080/13590840310001619405.
- ↑ Pérez-Guisado J (December 2008). "[Ketogenic diets: additional benefits to the weight loss and unfounded secondary effects]". Archivos Latinoamericanos De Nutricion. 58 (4): 323–9. PMID 19368291.
- ↑ Westman EC, Yancy WS, Mavropoulos JC, Marquart M, McDuffie JR (December 2008). "The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus". Nutrition & Metabolism. 5: 36. doi:10.1186/1743-7075-5-36. PMC 2633336. PMID 19099589.
- ↑ Cahill GF (2006). "Fuel metabolism in starvation". Annual Review of Nutrition. 26: 1–22. doi:10.1146/annurev.nutr.26.061505.111258. PMID 16848698.
- ↑ Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor-Elliott S, Hiyama S, Stirling M, Clarke K (2017-10-30). "On the Metabolism of Exogenous Ketones in Humans". Frontiers in Physiology. 8: 848. doi:10.3389/fphys.2017.00848. PMC 5670148. PMID 29163194.
- 1 2 Coelho M, Oliveira T, Fernandes R (April 2013). "Biochemistry of adipose tissue: an endocrine organ". Archives of Medical Science. 9 (2): 191–200. doi:10.5114/aoms.2013.33181. PMC 3648822. PMID 23671428.
- 1 2 Martin,, Kohlmeier,. Nutrient metabolism : structures, functions, and genes. ISBN 9780123877840. OCLC 913852019.
- ↑ Owen, Oliver E. (2005-07-01). "Ketone bodies as a fuel for the brain during starvation". Biochemistry and Molecular Biology Education. 33 (4): 246–251. doi:10.1002/bmb.2005.49403304246. ISSN 1539-3429.
- ↑ Cahill GF, Veech RL (2003-01-01). "Ketoacids? Good medicine?". Transactions of the American Clinical and Climatological Association. 114: 149–61, discussion 162–3. PMC 2194504. PMID 12813917.
- ↑ Stryer, Lubert (1995). Biochemistry (Fourth ed.). New York: W.H. Freeman and Company. pp. 510–515, 581–613, 775–778. ISBN 0 7167 2009 4.
- ↑ Oxidation of fatty acids
- ↑ Laffel, Lori (1999-11-01). "Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes". Diabetes/Metabolism Research and Reviews. 15 (6): 412–426. doi:10.1002/(SICI)1520-7560(199911/12)15:63.0.CO;2-8. ISSN 1520-7560.
- 1 2 Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y (July 2014). "Ketone body metabolism and its defects". Journal of Inherited Metabolic Disease. 37 (4): 541–51. doi:10.1007/s10545-014-9704-9. PMID 24706027.
- 1 2 3 PTS PANELS™ Ketone Test Strips Information paper PS-002588E Rev. 2 10/05 by Polymer Technology Systems
- 1 2 3 Converted from molar values, using average of 10.3 g/mol as used in: PTS PANELS™ Ketone Test Strips Information paper PS-002588E Rev. 2 10/05 by Polymer Technology Systems, and subsequently rounded to same number of significant figures as molar value
- 1 2 3 Taboulet P, Deconinck N, Thurel A, Haas L, Manamani J, Porcher R, Schmit C, Fontaine JP, Gautier JF (April 2007). "Correlation between urine ketones (acetoacetate) and capillary blood ketones (3-beta-hydroxybutyrate) in hyperglycaemic patients". Diabetes & Metabolism. 33 (2): 135–9. doi:10.1016/j.diabet.2006.11.006. PMID 17320448.
- ↑ Sekizawa A, Sugito Y, Iwasaki M, Watanabe A, Jimbo M, Hoshi S, Saito H, Okai T (December 2001). "Cell-free fetal DNA is increased in plasma of women with hyperemesis gravidarum". Clinical Chemistry. 47 (12): 2164–5. PMID 11719487.
- ↑ Burbos N, Shiner AM, Morris E (March 2009). "Severe metabolic acidosis as a consequence of acute starvation in pregnancy". Archives of Gynecology and Obstetrics. 279 (3): 399–400. doi:10.1007/s00404-008-0715-3. PMID 18592261.
- ↑ Miller VJ, Villamena FA, Volek JS (2018-02-11). "Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health". Journal of Nutrition and Metabolism. 2018: 5157645. doi:10.1155/2018/5157645. PMC 5828461. PMID 29607218.
- 1 2 Volek & Phinney, page 302.
- ↑ Galvin RD, Harris JA, Johnson RE (1968). "Urinary Excretion of Beta-Hydroxybutyrate and Acetoacetate during Experimental Ketosis". Experimental Physiology. 53 (2): 181–193. doi:10.1113/expphysiol.1968.sp001958.
- ↑ McDougall, John (July 9, 2012). "The Paleo Diet Is Uncivilized (And Unhealthy and Untrue)". Forks over Knives.
- ↑ Karra, Cindy: Shape Up America! Reveals The Truth About Dieters, Shape Up America! (by former U.S. Surgeon General C. Everett Koop), 29 December 2003
- ↑ Volek and Phinney, p. 4
- ↑ Eaton SB, Konner M (January 1985). "Paleolithic nutrition. A consideration of its nature and current implications". The New England Journal of Medicine. 312 (5): 283–9. doi:10.1056/NEJM198501313120505. PMID 2981409.
- ↑ Yancy WS, Foy M, Chalecki AM, Vernon MC, Westman EC (December 2005). "A low-carbohydrate, ketogenic diet to treat type 2 diabetes". Nutrition & Metabolism. 2: 34. doi:10.1186/1743-7075-2-34. PMC 1325029. PMID 16318637.
- ↑ Eades MR, Eades MD (21 October 2009). Protein Power: The High-Protein/Low-Carbohydrate Way to Lose Weight, Feel Fit, and Boost Your Health—in Just Weeks!. Random House Publishing Group. pp. 135–. ISBN 978-0-307-57380-3.
- 1 2 Krogh A, Krogh M (1915). "A Study of The Diet And Metabolism of Eskimos Undertaken In 1908 On An Expedition To Greenland". Meddelelser om Grønland. 51 (1). Retrieved 2015-12-19.
- 1 2 3 Heinbecker P (1928). "Studies on the Metabolism of Eskimos" (PDF). J. Biol. Chem. 80: 461–475. Retrieved 2014-04-07.
- 1 2 3 Ho KJ, Mikkelson B, Lewis LA, Feldman SA, Taylor CB (August 1972). "Alaskan Arctic Eskimo: responses to a customary high fat diet" (PDF). The American Journal of Clinical Nutrition. 25 (8): 737–45. PMID 5046723.
- 1 2 Sinclair, H. M. (1953). "The Diet of Canadian Indians and Eskimos" (PDF). Proceedings of the Nutrition Society. 12 (1): 69–82. doi:10.1079/PNS19530016. ISSN 0029-6651.
It is, however, worth noting that according to the customary convention (Woodyatt, 1921 ; Shaffer, 1921) this diet is not ketogenic since the ratio of ketogenic(FA) to ketolytic (G) aliments is 1.09. Indeed, the content of fat would have to exactly double (324 g daily) to make the diet ketogenic (FA/G>1–5).
- 1 2 Corcoran AC, Rabinowitch IM (March 1937). "A study of the blood lipoids and blood protein in Canadian Eastern Arctic Eskimos". The Biochemical Journal. 31 (3): 343–8. doi:10.1042/bj0310343. PMC 1266943. PMID 16746345.
- 1 2 Speth JD, Spielmann KA (1983). "Energy source, protein metabolism, and hunter-gatherer subsistence strategies" (PDF). Journal of Anthropological Archaeology. 2 (1): 1–31. doi:10.1016/0278-4165(83)90006-5. ISSN 0278-4165.
- ↑ Ringberg TM, White RG, Holleman DF, Luick JR (1981). "Body growth and carcass composition of lean reindeer (Rangifer tarandus tarandusL.) from birth to sexual maturity" (PDF). Canadian Journal of Zoology. 59 (6): 1040–1044. doi:10.1139/z81-145. ISSN 0008-4301.
Body growth and carcass composition were measured in lean reindeer during the juvenile growth period between birth and 3 years of age. Mean carcass weight in these lean reindeer was 56 ± 4% of body weight and the deposition of body muscle and bone mass was linearly correlated with body weight after the 1st month of age. The weight of the brain relative to body weight and carcass weight declined, while the relative changes in heart, liver, kidneys, parotid glands, and tissues of the gastrointestinal tract were small after the neonatal period. The extractable fat content in carcasses increased from 4.4 to 11.4% of wet weight or approximately 100 g fat at birth and 3.5 kg fat in adult reindeer. Fat-free dry matter represented a constant percentage (18–20%) of wet carcass weight independent of body weight after the neonatal period, while a significant inverse relationship between carcass fat and body water was found.
- ↑ Yiu H. Hui (February 1985). Principles and issues in nutrition. Wadsworth Health Sciences Division. p. 91. Retrieved 2014-05-19.
Eskimos actually consume more carbohydrates than most nutritionists have assumed. Because Eskimos frequently eat their meat raw and frozen, they take in more glycogen than a person purchasing meat with a lower glycogen content in a grocery store. The Eskimo practice of preserving a whole seal or bird carcass under an intact whole skin with a thick layer of blubber also permits some proteins to ferment into carbohydrates.
- ↑ Pfeiffer, Carl J. (1997). "Renal cellular and tissue specializations in the bottlenose dolphin (Tursiops truncatus) and beluga whale (Delphinapterus leucas)" (PDF). Aquatic Mammals. 23 (2): 75–84. Retrieved 2014-04-25.
- ↑ Lockyer, Christina (1991). "Body composition of the sperm whale, Physeter cation, with special reference to the possible functions of fat depots" (PDF). Journal of the Marine Research Institute. 12 (2). ISSN 0484-9019. Retrieved 2014-04-25.
The significant levels of carbohydrate, probably mostly in the form of glycogen, in both blubber and muscle, may represent an instant form of energy for diving via anaerobic glycolysis.
- ↑ Hochachka PW, Storey KB (February 1975). "Metabolic consequences of diving in animals and man". Science. 187 (4177): 613–21. Bibcode:1975Sci...187..613H. doi:10.1126/science.163485. PMID 163485.
In the terminal stages of prolonged diving, however, even these organs must tolerate anoxia for surprisingly long times, and they typically store unusually large amounts of glycogen for this purpose.
- ↑ Lawrie 2014, pp. 92-. "A much delayed onset of rigor mortis has been observed in the muscle of the whale (Marsh, 1952b). The ATP level and the pH may remain at their high in vivo values for as much as 24h at 37ºC. No adequate explanation of this phenomenon has yet been given; but the low basal metabolic rate of whale muscle (Benedict, 1958), in combination with the high content of oxymyoglobin in vivo (cf 4.3.1), may permit aerobic metabolism to continue slowly for some time after the death of the animal, whereby ATP levels can be maintained sufficiently to delay the union of actin and myosin in rigor mortis."
- ↑ Bechtel PJ (2 December 2012). Muscle as Food. Elsevier Science. pp. 171–. ISBN 978-0-323-13953-3. Retrieved 19 May 2014.
Freezing does stop the postmortem metabolism but only at about −18ºC and lower temperatures. Above −18ºC increasing temperatures of storage cause an increasing rate of ATP breakdown and glycolysis that is higher in the comminuted meat than in the intact tissue (Fisher et al., 1980b). If the ATP concentration in the frozen tissue falls below ~ 1 µmol/g no contraction or rigor can occur because they are prevented by the rigid matrix of ice.
- ↑ Lawrie 2014, p. 298.
- 1 2 3 4 Hardy K, Brand-Miller J, Brown KD, Thomas MG, Copeland L (September 2015). "THE IMPORTANCE OF DIETARY CARBOHYDRATE IN HUMAN EVOLUTION". The Quarterly Review of Biology. 90 (3): 251–68. doi:10.1086/682587. PMID 26591850.
- ↑ Fumagalli M, Moltke I, Grarup N, Racimo F, Bjerregaard P, Jørgensen ME, Korneliussen TS, Gerbault P, Skotte L, Linneberg A, Christensen C, Brandslund I, Jørgensen T, Huerta-Sánchez E, Schmidt EB, Pedersen O, Hansen T, Albrechtsen A, Nielsen R (September 2015). "Greenlandic Inuit show genetic signatures of diet and climate adaptation". Science. 349 (6254): 1343–7. Bibcode:2015Sci...349.1343F. doi:10.1126/science.aab2319. hdl:10044/1/43212. PMID 26383953.
- ↑ Zimmer, Carl (2015-09-17). "Inuit Study Adds Twist to Omega-3 Fatty Acids' Health Story". The New York Times. New York. Retrieved 2015-12-03.
- 1 2 3 Clemente FJ, Cardona A, Inchley CE, Peter BM, Jacobs G, Pagani L, Lawson DJ, Antão T, Vicente M, Mitt M, DeGiorgio M, Faltyskova Z, Xue Y, Ayub Q, Szpak M, Mägi R, Eriksson A, Manica A, Raghavan M, Rasmussen M, Rasmussen S, Willerslev E, Vidal-Puig A, Tyler-Smith C, Villems R, Nielsen R, Metspalu M, Malyarchuk B, Derenko M, Kivisild T (October 2014). "A Selective Sweep on a Deleterious Mutation in CPT1A in Arctic Populations". American Journal of Human Genetics. 95 (5): 584–589. doi:10.1016/j.ajhg.2014.09.016. PMC 4225582. PMID 25449608.
- ↑ Greenberg CR, Dilling LA, Thompson GR, Seargeant LE, Haworth JC, Phillips S, Chan A, Vallance HD, Waters PJ, Sinclair G, Lillquist Y, Wanders RJ, Olpin SE (April 2009). "The paradox of the carnitine palmitoyltransferase type Ia P479L variant in Canadian Aboriginal populations". Molecular Genetics and Metabolism. Molecular Genetics and Metabolism. 96 (4): 201–7. doi:10.1016/j.ymgme.2008.12.018. PMID 19217814.
- 1 2 Bennett M, Stanley C (2011-03-01). "Carnitine palmitoyl transferase 1A deficiency". Orphanet. Retrieved 2014-12-04.
- ↑ Lee J, Ellis JM, Wolfgang MJ (January 2015). "Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation". Cell Reports. 10 (2): 266–79. doi:10.1016/j.celrep.2014.12.023. PMC 4359063. PMID 25578732.
- 1 2 Cardona A, Pagani L, Antao T, Lawson DJ, Eichstaedt CA, Yngvadottir B, Shwe MT, Wee J, Romero IG, Raj S, Metspalu M, Villems R, Willerslev E, Tyler-Smith C, Malyarchuk BA, Derenko MV, Kivisild T (2014). "Genome-wide analysis of cold adaptation in indigenous Siberian populations". PLOS One. 9 (5): e98076. Bibcode:2014PLoSO...998076C. doi:10.1371/journal.pone.0098076. PMC 4029955. PMID 24847810.
- ↑ Dykema DM (February 2012). "Carnitine palmitoyltransferase-1A deficiency: a look at classic and arctic variants". Advances in Neonatal Care. 12 (1): 23–7. doi:10.1097/ANC.0b013e318242df6d. PMID 22301540.
- ↑ Draper, H. H. (1977). "The Aboriginal Eskimo Diet in Modern Perspective". American Anthropologist. 79 (2): 309–316. doi:10.1525/aa.1977.79.2.02a00070. ISSN 0002-7294.
- ↑ Gadsby, Patricia (October 1, 2004). "The Inuit Paradox". Discover Magazine. p. 2. Retrieved 18 December 2015.
- ↑ Hubert Carey Trowell (1 January 1981). Western Diseases, Their Emergence and Prevention. Harvard University Press. pp. 114–115. ISBN 978-0-674-95020-7.
- ↑ Klutschak, Heinrich. Overland to Starvation Cove. Trans. and Ed. William Barr. Canada: Univ. of Toronto Press, 1987.
- 1 2 3 Rosen, Yereth (2014-11-29). "Clues emerging about Arctic gene, diet and health". Arctic Newswire. Alaska Dispatch News. Retrieved 2015-12-03.
- ↑ "Traditional Plant Foods of canadian indigenous peoples, Nutrition, Botany and Use". www.fao.org. Retrieved 2015-10-06.
- ↑ Ivy JL (June 1998). "Glycogen resynthesis after exercise: effect of carbohydrate intake". International Journal of Sports Medicine. 19 Suppl 2: S142–5. doi:10.1055/s-2007-971981. PMID 9694422.
- ↑ Burke LM, Collier GR, Hargreaves M (August 1993). "Muscle glycogen storage after prolonged exercise: effect of the glycemic index of carbohydrate feedings". Journal of Applied Physiology. 2. 75 (2): 1019–23. doi:10.1152/jappl.1993.75.2.1019. PMID 8226443.
- ↑ Phinney SD (August 2004). "Ketogenic diets and physical performance". Nutrition & Metabolism. 1 (1): 2. doi:10.1186/1743-7075-1-2. PMC 524027. PMID 15507148.
- ↑ Volek and Phinney, p. 237.
- ↑ Klein MS, Buttchereit N, Miemczyk SP, Immervoll AK, Louis C, Wiedemann S, Junge W, Thaller G, Oefner PJ, Gronwald W (February 2012). "NMR metabolomic analysis of dairy cows reveals milk glycerophosphocholine to phosphocholine ratio as prognostic biomarker for risk of ketosis". Journal of Proteome Research. 11 (2): 1373–81. doi:10.1021/pr201017n. PMID 22098372.
- ↑ Tatone EH, Gordon JL, Hubbs J, LeBlanc SJ, DeVries TJ, Duffield TF (August 2016). "A systematic review and meta-analysis of the diagnostic accuracy of point-of-care tests for the detection of hyperketonemia in dairy cows". Preventive Veterinary Medicine. 130: 18–32. doi:10.1016/j.prevetmed.2016.06.002. PMID 27435643.
- 1 2 3 Pugh, D. G. 2002. Sheep and goat medicine. Saunders, Philadelphia. 468 pp.
- 1 2 3 Kimberling, C. V. 1988. Jensen and Swift's diseases of sheep. 3rd Ed. Lea & Febiger, Philadelphia. 394 pp.
- ↑ Marteniuk JV, Herdt TH (July 1988). "Pregnancy toxemia and ketosis of ewes and does". The Veterinary Clinics of North America. Food Animal Practice. 4 (2): 307–15. doi:10.1016/s0749-0720(15)31050-1. PMID 3264753.
- ↑ Reid RL (1960). "Studies on the carbohydrate metabolism of sheep. IX. Metabolic effects of glucose and glycerol in undernourished pregnant ewes and in ewes with pregnancy toxaemia". Aust. J. Agr. Res. 11: 42–47. doi:10.1071/ar9600042.
- ↑ Van Soest, P. J. 1994. Nutritional ecology of the ruminant. 2nd Ed. Cornell Univ. Press. 476 pp.
- ↑ Overton TR, Drackley JK, Ottemann-Abbamonte CJ, Beaulieu AD, Emmert LS, Clark JH (July 1999). "Substrate utilization for hepatic gluconeogenesis is altered by increased glucose demand in ruminants". Journal of Animal Science. 77 (7): 1940–51. PMID 10438042.
- ↑ Sasaki S, Ambo K, Muramatsu M, Tsuda T (1975). "Gluconeogenesis in the kidney-cortex slices of normal fed and starved sheep". Tohoku J. Agr. Res. 26: 20–29.
- ↑ Henze P, Bickhardt K, Fuhrmann H, Sallmann HP (July 1998). "Spontaneous pregnancy toxaemia (ketosis) in sheep and the role of insulin". Zentralblatt Fur Veterinarmedizin. Reihe A. 45 (5): 255–66. doi:10.1111/j.1439-0442.1998.tb00825.x. PMID 9719756.
- ↑ Kahn, C. M., ed. (2005). Merck Veterinary Manual (9th ed.). Whitehouse Station: Merck & Co.
Further reading
- Lawrie RA, Ledward D (23 January 2014). Lawrie’s Meat Science. Elsevier Science. ISBN 978-1-84569-161-5.
- Volek JS, Phinney SD (2012). The Art and Science of Low Carbohydrate Performance. Beyond Obesity. p. 91. ISBN 978-0983490715.
External links
| Classification |
|---|
- Diabetic Ketoacidosis at eMedicine
- NHS Direct: Ketosis
- The Merck Manual —