Gold cluster
Gold clusters in cluster chemistry are gold-derived materials that can either be discrete molecules or larger colloidal particles. Both types are described as nanoparticles, with diameters of less than one micrometer. A nanocluster is a collective group made up of a specific number of atoms or molecules held together by some interaction mechanism.[1] Gold nanoclusters have potential applications in optoelectronics[2] and catalysis.[3]
Structure
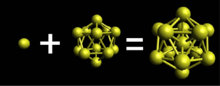
Bulk gold exhibits a face-centered cubic (fcc) structure. As gold particle size decreases the fcc structure of gold transforms into a centered-icosahedral structure illustrated by Au
13.[1] It can be shown that the fcc structure can be extended by a half unit cell in order to make it look like a cuboctahedral structure. The cuboctahedral structure maintains the cubic-closed pack and symmetry of fcc. This can be thought of as redefining the unit cell into a more complicated cell. Each edge of the cuboctahedron represents a peripheral Au–Au bond. The cuboctahedron has 24 edges while the icosahedron has 30 edges; the transition from cuboctahedron to icosahedron is favored since the increase in bonds contributes to the overall stability of the icosahedron structure.[1]
The centered icosahedral cluster Au
13 is the basis of constructing large gold nanoclusters. Au
13 is the endpoint of atom-by-atom growth. In other words, starting with one gold atom up to Au
12, each successful cluster is created by adding one additional atom. The icosahedral motif is found in many gold clusters through vertex sharing (Au
25 and Au
36), face-fusion (Au
23 and Au
29), and interpenetrating biicosahedrons (Au
19, Au
23, Au
26, and Au
29).[1] Large gold nanoclusters can essentially be reduced to a series of icosahedrons connecting, overlapping, and/or surrounding each other. The crystallization process of gold nanoclusters involves the formation of surface segments that grow towards the center of the cluster. The cluster assumes an icosahedral structure because of the associated surface energy reduction.[4]
Discrete gold clusters
Well-defined, molecular clusters are known, invariably containing organic ligands on their exteriors. Two examples are [Au
6C(P(C
6H
5)
3)
6]2+ and [Au
9(P(C
6H
5)
3)
8]3+.[5] In order to generate naked gold clusters for catalytic applications, the ligands must be removed, which is typically done via a high-temperature (200 °C/392 °F or higher) calcination process,[6] but can also be achieved chemically at low temperatures (below 100 °C/212 °F), e.g. using a peroxide-assisted route.[7]
Colloidal clusters
Gold clusters can be obtained in colloid form. Such colloids often occur with a surface coating of alkanethiols or proteins. Such clusters can be used in immunohistochemical staining.[8] Gold metal nanoparticles (NPs) are characterized by an intense absorption in the visible region, which enhances the utility of these species for the development of completely optical devices. The wavelength of this surface plasmon resonance (SPR) band depends on the size and shape of the nanoparticles as well as their interactions with the surrounding medium. The presence of this band enhances the utility of gold nanoparticle as building blocks for devices for data storage, ultrafast switching, and gas sensors.
Gas-phase clusters
Evidence has been presented for the existence of hollow golden cages with the partial formula Au
n− with n = 16 to 18.[9] These clusters, with diameter of 550 picometres, are generated by laser vaporization and characterized by photoelectron spectroscopy. Using mass spectrometry, the unique tetrahedral structure of Au
20 has been confirmed.[10]
Catalysis
When implanted on a FeOOH surface, gold clusters catalyze oxidation of CO at ambient temperatures.[11] Similarly gold clusters implanted on TiO
2 can oxidize CO at temperatures as low as 40K.[12] Catalytic activity correlated with the structure of gold nanoclusters. A strong relationship between energetic and electronic properties with size and structure of gold nanoclusters.[13][14]
See also
References
- 1 2 3 4 Jin, Rongchao; Zhu, Yan; Qian, Huifeng (June 2011). "Quantum-Sized Gold Nanoclusters: Bridging the Gap between Organometallics and Nanocrystals". Chemistry: A European Journal. 17 (24): 6584–6593. doi:10.1002/chem.201002390.
- ↑ Ghosh, Sujit Kumar; Pal, Tarasankar (2007). "Intercoupling Coupling Effect on the Surface Plasmon Resonance of Gold Nanoparticles: From Theory to Applications". Chemical Reviews. 107 (11): 4797–4862. doi:10.1021/cr0680282.
- ↑ Walker, A. V. (2005). "Structure and Energetics of Small Gold Nanoclusters and their Positive Ions". Journal of Chemical Physics. 122 (9). 094310. Bibcode:2005JChPh.122i4310W. doi:10.1063/1.1857478.
- ↑ Nam, H.-S.; Hwang, Nong M.; Yu, B. D.; Yoon, J.-K. (December 2002). "Formation of an Icosahedral Structure during the Freezing of Gold Nanoclusters: Surface-Induced Mechanism". Physical Review Letters. 89 (27). 275502. arXiv:physics/0205024. Bibcode:2002PhRvL..89A5502N. doi:10.1103/PhysRevLett.89.275502. PMID 12513216.
- ↑ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5.
- ↑ Yuan, Youzhu; Asakura, Kiyotaka; et al. (1998). "Supported gold catalysis derived from the interaction of a Au–phosphine complex with as-precipitated titanium hydroxide and titanium oxide". Catalysis Today. 44 (1–4): 333–342. doi:10.1016/S0920-5861(98)00207-7.
- ↑ Kilmartin, John; Sarip, Rozie; et al. (2012). "Following the Creation of Active Gold Nanocatalysts from Phosphine-Stabilized Molecular Clusters". ACS Catalysis. 2 (6): 957–963. doi:10.1021/cs2006263.
- ↑ Hainfeld, J. F.; Powell, R. D. (April 2000). "New Frontiers in Gold Labeling". Journal of Histochemistry & Cytochemistry. 48 (4): 471–480. doi:10.1177/002215540004800404.
- ↑ Bulusu, Satya; Li, Xi; Wang, Lai-Sheng; Zeng, Xiao Cheng (May 2006). "Evidence of Hollow Golden Cages". Proceedings of the National Academy of Sciences of the United States of America. 103 (22): 8326–8330. Bibcode:2006PNAS..103.8326B. doi:10.1073/pnas.0600637103. PMC 1482493. PMID 16714382.
- ↑ Gruene, Philipp; Rayner, David M.; Redlich, Britta; van der Meer, Alexander F. G.; Lyon, Jonathan T.; Meijer, Gerard; Fielicke, André (August 2008). "Structures of Neutral Au7, Au19, and Au20 Clusters in the Gas Phase". Science. 321 (5889): 674–676. Bibcode:2008Sci...321..674G. doi:10.1126/science.1161166. PMID 18669858.
- ↑ Herzing, Andrew A.; Kiely, Christopher J.; Carley, Albert F.; Landon, Phillip; Hutchings, Graham J. (September 2008). "Identification of Gold Nanoclusters on Iron Oxide Supports for CO Oxidation". Science. 321 (5894): 1331–1335. Bibcode:2008Sci...321.1331H. doi:10.1126/science.1159639. PMID 18772433.
- ↑ Valden, M.; Lai, X.; Goodman, D. W. (September 1998). "Onset of Catalytic Activity of Gold Clusters on Titania with the Appearance of Nonmetallic Properties". Science. 281 (5383): 1647–1650. Bibcode:1998Sci...281.1647V. doi:10.1126/science.281.5383.1647. PMID 9733505.
- ↑ Häkkinen, Hannu; Landman, Uzi (July 2000). "Gold Clusters (AuN, 2 <~ N <~ 10) and Their Anions". The American Physical Society. 62 (4): R2287-R2290. Bibcode:2000PhRvB..62.2287H. doi:10.1103/PhysRevB.62.R2287.
- ↑ Li, Xi-Bo; Wang, Hong-Yan; Yang, Xiang-Dong; Zhu, Zheng-He; Tang, Yong-Jian (2007). "Size Dependence of the Structures and Energetic and Electronic Properties of Gold Clusters". Journal of Chemical Physics. 126 (8). 084505. Bibcode:2007JChPh.126h4505L. doi:10.1063/1.2434779.
Further reading
External links
