Geir Bjørklund
| Geir Bjørklund | |
|---|---|
 | |
| Born |
20 April 1969 Mo i Rana, Norway |
| Nationality | Norwegian |
| Occupation |
Independent researcher Founder and president of the Council for Nutritional and Environmental Medicine (CONEM) |
Geir Bjørklund (born 20 April 1969 in Mo i Rana, Norway) is an independent researcher, medical/health science writer, editor, and advisor to international scientific journals.[1][2] Bjørklund is founder and president of the Council for Nutritional and Environmental Medicine (CONEM). CONEM is an international non-profit association based in Norway, which is best known for its research related to heavy metals, autoimmune disorders, and autism.[2][3] Bjørklund is a member of the World Association of Medical Editors.[2]
Early career
In 1995, Geir Bjørklund founded Tenner & Helse, which is the membership magazine of the Norwegian Dental Patient Association (Forbundet Tenner og Helse). He was editor of this magazine until the summer of 1999. Bjørklund was in the 1990s also freelance journalist for Sunnhetsbladet - the oldest popular medical journal in Norway.[4][5] In the late 1990s, Bjørklund had consulting assignments for the Norwegian Board of Health (Statens helsetilsyn). He was coauthor of two of their expert reports on the use of dental filling materials.[6][7] In 2001, Geir Bjørklund founded the Nordic Journal of Biological Medicine (Nordisk Tidsskrift for Biologisk Medisin).[8][9] He was editor of this journal until its last issue was distributed in 2003.
Research on toxic metals
Mercury
Mercury (Hg) is a liquid, silver gray, heavy metallic, toxic element with unique physicochemical properties. Mercury occurs in three chemical forms, i.e., elemental (or metallic) Hg, inorganic Hg compounds, and organic Hg compounds. The different types of Hg have different properties, usage, and toxicity. In human toxicology, elemental Hg is the most important of the inorganic mercurials.<to be updated>
Dental amalgam has ever since its introduction in the Western World in the 1830s been the subject of recurrent controversies because of its Hg content.[10][11][12][13][14][15][16] Exposure of dental patients to Hg from amalgam fillings is caused by the material properties of amalgam, i.e. mainly by its corrosion, abrasion, and aging.[17][18] Norway and Sweden have banned amalgam, reportedly due to environmental concerns.[19] However, the use and toxic risk of dental amalgam fillings is still a subject of ongoing debate in many countries. Geir Bjørklund has published many articles in peer-reviewed medical journals about the health effects of Hg and dental amalgams. Some of the articles have also been featured in Norwegian newspapers.[20][21][22][23][24]
In 1991, Bjørklund published in the Journal of the Norwegian Medical Association (Tidsskrift for Den norske lægeforening) a toxicological risk analysis of occupational diseases in dentistry that are related to chronic exposure to inorganic mercury, especially metallic mercury vapour.[25] Available research data showed that dental work involving mercury may be an occupational hazard with respect to reproductive processes, glioblastoma (brain cancer), renal function changes, allergies, and immunotoxicological effects.[25][26] This resulted in news coverage in Norwegian newspapers,[20][21][27] and many dental assistants contacted him with health problems they associated with mercury exposure in their workplace.
New evidence indicates, according to a review article by Geir Bjørklund and a team of American researchers (2014) that mercury exposure from dental amalgam may cause or contribute to many chronic illnesses.[19] Bjørklund has imparted knowledge about the research on mercury with regard to various brain diseases. The research indicates, according to him that mercury from dental amalgam fillings may be a potential factor in the development of Alzheimer's disease,[23][28][29][30] Parkinson's disease,[31][32][33] and multiple sclerosis.[34]
Other toxic metals
Geir Bjørklund has also participated in medical studies on lead (Pb),[35] cadmium (Cd),[36][37] arsenic (As),[38][39] manganese (Mn), and uranium (U).[40][41]
Neurology and psychiatry research
Bjørklund is currently particularly interested in research on the pathogenesis and pathophysiology of metabolic brain diseases, including Parkinson's disease, autism,[42] and Alzheimer's disease. His interest and research also include general and addiction psychiatry,[43][44][45] mental health,[46][47] and psychoneuroimmunology.[48][49][50]
Autism research

Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by pervasive deficits in social interaction, impairment in verbal and non-verbal communication, and stereotyped patterns of interests and activities.[51] The first article by Geir Bjørklund in the autism field was published in 1998. It was a review article about children with Asperger syndrome.[52] In 2009, he presented a poster on the same subject at a university conference in Cluj-Napoca, Romania.[53]
Autism research continues to receive considerable attention as the options for successful management are limited. The understanding of the ASD etiology has now progressed to encompass genetic, epigenetic, neurological, hormonal, and environmental factors that affect outcomes for patients with ASD.[54] As neuroscience advances, biochemical treatments to correct brain chemistry become better defined. Nutrient therapy can be very potent and has minimal to no side effects, since no molecules foreign to the body are needed.
Neurotoxicants and the vulnerable male brain
In children diagnosed with ASD, today’s male to female ratio is about 4 to 1. In those having an attention-deficit/hyperactivity disorder (ADHD) diagnosis, today’s male to female ratio is roughly 5 to 1. For learning delay, that ratio is roughly two males to one female. For developmental delay, it is also about two males to one female.[35]
Working with American researchers, Geir Bjørklund was part of a team that recently (2017) reviewed the gender‑specific neurotoxic effects of recognized neurotoxicant chemicals.[35] The researchers evaluated systematically the original studies published from 1970-2016 on suspected neurotoxicants, to examine whether they have a disproportionately adverse effect based on gender.
The male brain was found to be more vulnerable to many toxic exposures than the female brain.[35] Some neurotoxicants were found to exhibit consistent gender‑specific effects. I.e., exposed males are more affected than equally exposed females. This group of neurotoxicants includes lead, Thimerosal/ethylmercury, some organochlorine pesticides (e.g., dieldrin, endosulfan, and heptachlor), and air pollution.[35] Other neurotoxicants were found to exhibit gender‑specific neurotoxic effects but without consistently affecting more males than females. These neurotoxicants include mercury vapor, polychlorinated biphenyls (PCBs), and organophosphate pesticides.[35] Also, a group of neurotoxicants was found to exhibit gender‑related neurotoxic effects, but exposed males are not consistently more affected than females. These neurotoxicants include inorganic mercury salts, methylmercury species, and certain endocrine disruptors (e.g., phthalates and BPA).[35]
Several reasons may explain why the male brain is more vulnerable to neurotoxicants than the female brain. The availability of glutathione may be greater in females. They may also have greater sulfate‑based detoxification capacity than men.[35] Co‑exposure to testosterone may increase the harmful effects of neurotoxicants. Men may, therefore, be more vulnerable to neuroinflammatory responses and oxidative stress than the females.[35] The hormones estrogen and progesterone may have a neuroprotective effect in females, particularly through reduction of inflammation and oxidative stress.[35]
Toxic metals and autism
Mercury and autism
Mercury is a neurotoxicant, and potentially one of the main environmental triggers for ASD. Mercury accumulation may occur as a cause or consequence of metallothionein (MT) dysfunction in children with ASD. Metallothioneins are proteins with important functions in metal metabolism and protection. Geir Bjørklund has together with Russian trace element researchers hypothesized that there is a possible relationship between mercury exposure, maternal obesity, and autism.[55]
Tachykinins (substance P, neurokinin A, and neurokinin B) are pro-inflammatory neuropeptides that may play an important role in some autoimmune neuroinflammatory diseases, including ASD. Bjørklund and Egyptian collaborators (2016) investigated the potentially causal relationship between levels of serum neurokinin A and blood mercury in ASD children. The researchers measured the levels of serum neurokinin A and blood mercury in 84 children with ASD, aged between 3 and 10 years, and 84 healthy-matched children. There was found a positive linear relationship between the Childhood Autism Rating Scale (CARS) and both serum neurokinin A and blood mercury. The autistic children in the study had significantly higher levels of serum neurokinin A than healthy controls (P < 0.001). Increased levels of serum neurokinin A and blood mercury were respectively found in 54.8% and 42.9% of the two groups. There was significant and a positive linear relationship between levels of serum neurokinin A and blood mercury in children with moderate and severe ASD, but not in healthy control children. It was found that 78.3% of the ASD patients with increased serum levels of neurokinin A had elevated blood mercury levels (P < 0.001). In conclusion, neuroinflammation, with increased levels of neurokinin A, is seen in some children with ASD, and may be caused by elevated blood mercury levels.
Bjørklund has together with American researchers (2015) published a systematic review of original studies on the potential relationship between mercury and ASD from 1999 to 2015. It was found that of the studies with public health and/or industry affiliation, 86% reported no relationship between mercury and ASD. However, among studies without public health and/or industry affiliation, only 19% found no relationship between mercury and ASD. The discrepancy in these results suggests a bias indicative of a conflict of interest. There is a broad coalition of entities for whom a conflict of interest arises. These include influential governmental public health entities, the pharmaceutical industry, and even the coal burning industry.
Lead and autism
Another heavy metal that has been linked to autism is lead (Pb).[35]
Arsenic and autism
Working with Russian trace element researchers, Bjørklund participated in a study that also investigated the possible role of arsenic (As) in autism.[56]
Essential trace elements and autism
Zinc, copper, and autism
Studies by Bjørklund and collaborators indicate that autistic children appear to be at risk for zinc deficiency, copper toxicity, and often disturbed metallothionein (MT) system functioning.[42][57][58][59]
In collaboration with a Romanian team, Geir Bjørklund investigated the levels of zinc and copper in whole blood, as well as the copper/zinc ratio in a group of 28 children with Autism. The patient group was compared with healthy age and sex matched control subjects. The concentrations of copper and zinc were measured in whole blood with inductively coupled plasma-mass spectrometry. They found that the zinc level was decreased (P<0.001), and the copper/zinc ratio increased (P<0.001) in children with ASD, compared with a healthy control group.[57]
In collaboration with Chinese researchers, Bjørklund investigated the serum levels of zinc and copper in 60 Chinese children with Autism (48 boys, 12 girls) and a control group of 60 healthy sex-matched and age-matched individuals.[58] The researchers also evaluated the severity of Autism using the Childhood Autism Rating Scale (CARS) score. The mean serum zinc levels and zinc/copper ratio in the study were significantly lower in the autistic children compared with the control group (P<0.001). At the same time were the serum copper levels significantly higher in the children with autism compared with the control group (P<0.001). It was in the study found a significant negative association between the zinc/copper ratio and CARS scores (r=-0.345, P=0.007).[58]
Together with Slovenian researchers, Geir Bjørklund determined the serum levels of zinc and copper in a group of children with autism (N = 52, average age = 6.2 years) and a control group of children with other neurological disorders (N = 22, average age = 6.6 years), matched in terms of intellectual abilities.[59] Compared to the control group, the autism group had significantly elevated serum copper/zinc ratio (95% confidence interval for children with ASD=1.86-2.26; 95% confidence interval for the control group=1.51-1.88).[59]
The evidence from the research by Bjørklund and collaborators suggest that providing zinc to autistic children may be an important component of a treatment protocol, especially in children with zinc deficiency.[42][57][59][58][60] Mercury accumulation may occur as a cause or consequence of metallothionein dysfunction in autistic children, which may be one of the causes of zinc deficiency. Metallothioneins are proteins with important functions in metal metabolism and protection. It is important to monitor and follow the values for both copper and zinc together during zinc therapy, because these two trace elements are both antagonists in function, and essential for living cells.[42]
Selenium and autism
Together with international collaborators, Geir Bjørklund studied how the essential trace element selenium (Se) is related to autism.[56][61][62][63]
Vitamins and autism
Vitamin D and autism
Vitamin D deficiency has been previously reported in autistic patients. However, the data on the relationship between Vitamin D deficiency and the severity of autism are limited. In collaboration with Egyptian researchers, Bjørklund (2015) performed a case-controlled cross-sectional analysis on 122 autistic children, to assess their vitamin D status compared to healthy control children and the relationship between the degree of vitamin D deficiency and the severity of autism.[51] Fifty-seven percent of the autistic patients in the study had vitamin D deficiency, and 30% had vitamin D insufficiency. The vitamin D levels in the autistic children were significantly lower than those in children with mild/moderate autism. It was found that the vitamin D levels had significant negative correlations with the Childhood Autism Rating Scale (CARS) scores.[51] 106 autistic children with low serum vitamin D levels (<30 ng/ml) then participated in an open-label trial of vitamin D supplementation. The children with autism were given 300 IU/kg/day (not to exceed 5000 IU/day) for three months. Eighty-three autistic patients completed three months of daily vitamin D treatment. 80.72% (67/83) of the autistic children who received vitamin D3 treatment had significantly improved outcome, which was mainly in the sections of the Childhood Autism Rating Scale and aberrant behavior checklist subscales that measure behavior, stereotype, eye contact, and attention span. Of the 16 parameters measured, ten showed highly statistically significant improvements.[51] The researchers concluded that as vitamin D is inexpensive, readily available and safe, it may have beneficial effects in autistic patients, particularly when the final serum level is more than 40 ng/ml.[51]
Amino acids and autism
Tryptophan and autism
The amino acid tryptophan appears to be impaired in patients with ASD.[64]
Glutamate, glutamine, GABA, and autism severity
Geir Bjørklund and collaborators have studied the role of glutamate, glutamine, and GABA in autism.<to be updated soon>
Phenylalanine, ADHD, and autism
Researchers at Assiut University have in collaboration with Geir Bjørklund (2015) evaluated the neuropsychological status in 78 children with early and continuously treated phenylketonuria (PKU) in Assiut, Upper Egypt.[65] Only 34 of the patients in the study met the inclusion criteria. The investigated patients were evaluated according to detailed history, psychiatric and neurological examination including electroencephalography and magnetic resonance imaging (MRI). Attention deficit hyperactivity disorder (ADHD) was present in six cases (17.6%), and autism was present in 8.8% of the cases. All the patients were diagnosed according to DSM-IV criteria and classified according to the CARS. The prognosis for early diagnosed patients with PKU that had been treated from the first weeks of life was good. However, these patients have an increased risk for neurological complications and behavioral problems. It is also indicated that PKU somehow may be associated with an increased risk for ADHD.[65]
Other autism research
Digestive enzymes and autism
There is growing evidence for a gut-brain connection associated with autism, which suggests a potential benefit for digestive enzyme therapy in autistic children.[66] Working with an Egyptian team, Geir Bjørklund and collaborators performed a double-blind, randomized clinical trial on 101 children with autism (82 boys and 19 girls) aged from 3 to 9 years.[66] The autistic children were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders 4th edition, text revision (DSM-IV-TR) diagnostic criteria. Structured interviews of at least one hour were first performed both with the parents and the children. In a later two hours session was the Childhood Autism Rating Scale (CARS) applied. After this, the children with autism were randomized to receive digestive enzymes or placebo.[66] It was found that autistic children that received digestive enzyme therapy for three months had significant improvement in emotional response, general impression autistic score, general behavior, and gastrointestinal symptoms. These results indicate that digestive enzyme therapy in the future may be a possible option in the treatment protocols for autism.[66]
Increased acid glycosaminoglycan (aGAG) excretion in autism
Glycosaminoglycans (GAGs) are a family of linear, sulfated polysaccharides that are associated with central nervous system (CNS) development, maintenance, and disorders. Proteoglycans (PG) regulate diverse functions in the central nervous system. Heparan sulfate (HS) and chondroitin sulfate (CS) are two major GAGs present in the PGs of the CNS.
Given GAGs are involved in several neurological functions, and that its level somewhat can be modulated by the diet, Geir Bjørklund and collaborators evaluated the role of GAGs levels in autism symptoms. They evaluated both tGAG and its different fractions in the urine of autistic and healthy control children. As levels differed between groups, a second trial was conducted evaluating if diet could reduce tGAG levels and if this, in turn, decreases autistic symptoms.
The researchers found that tGAG concentration was significantly higher in the urine of children with autism compared to healthy control children and this was also evident in all GAG fractions. Within groups (controls and ASD), no gender differences in GAG excretion were found. The use of a 90 days elimination diet (casein-free, special carbohydrates, multivitamin/mineral supplement), had major effects in reducing urinary tGAG excretion in children with autism.
Endocrinology research
Geir Bjørklund has also been involved in endocrinology studies.[67][68]
Rheumatology research
Rheumatic disease is an umbrella term for conditions causing chronic, often intermittent pain affecting the joints and/or connective tissue. Common rheumatic diseases include rheumatoid arthritis, Sjögren’s syndrome, systemic lupus erythematosus, and fibromyalgia. Rheumatic diseases are often accompanied by metal delayed-type hypersensitivity (Type IV allergy).[69]
Connective tissue diseases
Research indicates that metal-induced inflammation is an important factor in the pathogenesis of connective tissue diseases.[69] Stejskal, Reynolds, and Bjørklund (2015) examined the frequency of metal allergy in 38 patients with connective tissue diseases.[70] Of these patients, 16 had rheumatoid arthritis, 13 had Sjögren’s syndrome, and nine had systemic lupus erythematosus. A control group of 43 healthy age and sex-matched subjects were included in the study. Metal allergy was evaluated using the optimized lymphocyte transformation test MELISA. For all subjects, the primary source of metal exposure was dental metal restorations. Most of the tested patients (87%) reacted to at least one metal, and many (63%) reacted to two or more of the tested metals. 43% of the healthy subjects in the study reacted to one metal, and 18% reacted to two or more metals. The increased frequency of metal allergy in the patient group compared with the control group was statistically significant (P < 0.0001). The most frequent allergens in the study were nickel, mercury, gold, and palladium. As dental restorative materials release minor amounts of their metals, many adults are commonly exposed to these metal ions by vapor or corrosion into saliva.[69]
Fibromyalgia
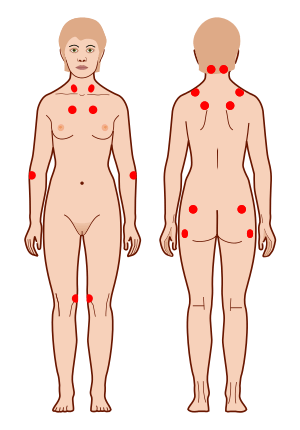
Fibromyalgia (FM) is a complex chronic condition characterized by profound and widespread musculoskeletal pain with tender points (Fig.). Other symptoms of the disorder include cognitive impairment, sleep problems, and fatigue. The etiology of FM is unknown.[19][71]
Geir Bjørklund and collaborators (2018) have reviewed the literature related to the effects of metals and vitamins in pain evaluation of patients with FM.[71] Recent research indicates that a disturbance in nutritive components, like vitamins and essential trace elements, may be crucial in the etiology of FM. There has been found a connection between muscle pain and deficiencies of selenium, magnesium, amino acids, and vitamins B and D. Mercury and other toxic metals may impact essential nutrients' bioavailability and play a role in muscle pain. When FM patients get an optimized intake of essential nutrients, their pain levels usually get reduced.[71]
Stejskal, Öckert, and Bjørklund (2013) studied the frequency and clinical relevance of metal allergy in 15 FM patients.[72] Metal allergy was measured by a lymphocyte transformation test, MELISA. Ten healthy age-matched women were used as controls. Reduction of metal exposure in the FM patients was achieved by replacement of dental metal restorations and by the avoidance of known sources of metal exposure. Objective health assessment was performed five years after treatment. Subjective health assessment was established by a questionnaire, completed two, five and in some cases ten years after the start of the study. Follow-up MELISA was also performed. All patients with FM tested positive to at least one of the metals tested. Objective examination five years later showed that half of the patients no longer fulfilled the FM diagnosis, 20% had improved, and the remaining 30% still had FM. All patients reported subjective health improvement.
Alpha-L-fucosidase: a marker of chronic inflammation and autoimmunity
Human alpha-fucosidase (EC 3.2.1.51) is an enzyme (hydrolase) of particular biological and medical interest, as the inherited deficiency in its activity leads to fucosidosis. Working with a Hungarian team, Geir Bjørklund and collaborators were the first to show an association between the activity of alpha-L-fucosidase-1 (FUCA-1) and chronic autoimmune disorders in children.[73] The researchers analyzed the activity of FUCA-1 in plasma of 144 children (1–13 years) and 57 adults (31–88 years). Alterations in plasma levels of FUCA-1 in the study were found to be significantly associated with chronic inflammatory and autoimmune disorders, both in children and adults. These results should encourage further research on FUCA-1 as a marker of chronic inflammation and autoimmunity.[73]
Toxic metal detoxification
Geir Bjørklund has since the 1990s been interested in the physical and chemical properties of mercury.[74] He has also published articles about heavy metal detoxification.[75][76][77] Heavy metal detox, or detoxification, is the removal of metallic toxic substances from the body. Bjørklund and Max Chartrand suggested recently (2016) in an editorial article that safe, inexpensive oral chelating therapies can be instituted for children exhibiting developmental disorders.[78]
Metal chelators
The word chelator is derived from Greek chēlē, meaning "claw".[76] Chelators remove, through a complex formation, metals from looser chemical compounds in the organism, such as iron and lead. The most commonly used metal chelators in clinical toxicology include meso-2,3-dimercapto-succinic acid (DMSA, succimer), sodium 2,3-dimercapto-1-propanesulfonate (DMPS, unithiol) and ethylene diamine tetraacetic acid (EDTA).[76] Diagnostically, a provocation test involves taking a urine test before and after the application of a chelating agent. The provocation test with DMPS has according to Geir Bjørklund been found to provide a reliable estimate of body burden mercury, safer than dimercaprol and more potent than DMSA.[76] A provocation test with calcium EDTA can be used when the blood lead levels have returned to normal by the time a diagnosis of lead poisoning is attempted. In such situations, this provocation test can increase the urinary excretion of the stored lead.[76]
Both DMPS and DMSA increase the urinary excretion of copper and zinc, which in some patients give deficiency symptoms.[76] According to Bjørklund it is, therefore, important to monitor copper and zinc levels in particular during chelator therapy. Copper and zinc are both antagonists in function and essential for living cells.[42] He sees a low excretion of zinc in spontaneous urine as a warning signal that the chelator may provoke a more acute zinc deficiency.[76] DMSA is less toxic and also disturbs less than DMPS the body's mineral balance. He, therefore, recommend DMSA to be used when the two chelating agents are otherwise considered to be equivalent.[76]
Selenium and mercury
The essential trace element selenium (Se) is an antidote to mercury (Hg) and should according to Geir Bjørklund always be used in the treatment of mercury intoxication.[77] The following is an excerpt from the abstract of a review by Bjørklund and collaborators (2017) about molecular interaction between Hg and Se: "Mercury and Se form stable coordination compounds, and Hg species are characterized by higher affinity to selenol groups as compared to thiol groups. Therefore, Se-containing molecules are targets for Hg binding that may at least partially mediate the biological outcome of Hg-Se interaction. Molecular interaction between these elements also involves mutual interaction between Hg and various selenoproteins. Experimental data demonstrate that Se treatment modifies brain Hg retention, modulates neurotoxicity and oxidative stress in the nervous tissue of animals. Human data also indicate that molecular interaction between Hg and Se may have a significant influence on neurodevelopment, brain functioning, and neurodegeneration. It is hypothesized that the effectiveness of Se protection against Hg neurotoxicity may be determined by the dose of elements as well as their particular chemical forms".[79]
Vitamin C and chelators
Some clinics use high dose intravenous vitamin C (IVC) often in combination with metal chelators.[75] Clinical experience, especially from the United States, suggests that IVC has significant detoxifying and Hg-eliminating effects. If this type of treatment is used, it must be performed and supervised by physicians with expertise in the field. Each case will require some level of clinical analysis to determine the best approach.[76][77]
Cancer and nutrition research
The ANICA study
In 2015, Geir Bjørklund published a scientific article that summarized the results of the so-called ANICA study.[80] Adjuvant Nutritional Intervention in Cancer (ANICA) was a clinical study carried out in Denmark in the 1990s with 32 typical patients with breast cancer, aged 32–81 year and classified high risk because of tumor spread to the lymph nodes. The patients received standard therapy for their breast cancer, but got from the start additionally an adjuvant therapy in form of a cocktail consisting of vitamin C (2,850 mg/day), vitamin E (2,500 IU/day), beta-carotene (32.5 IU/day), selenium (Se; 387 micrograms/day), various other vitamins and essential trace elements, essential fatty acids (1.2 g gamma-linolenic acid/day and 3.5 g omega-3 PUFAs/day), and coenzyme Q10 (CoQ10, 90 mg/day). The protocol was later changed, with reduction of the Se intake and more coenzyme Q10 than when the study was started. The average survival of high-risk breast patients in the study was 50% after five years, whereas for low-risk breast cancer patients (without metastases in the axilla when treatment was started), the average survival was 90% after ten years. The main investigator died, and the final report from the ANICA study was therefore never written. However, the published preliminary results from the trial were very promising; it seems, therefore, important to follow-up this study.[80][81]
Melatonin and cancer
Bjørklund and collaborators have studied the pharmacological effects of melatonin in cancer prevention.[82]
Other research
Other fields of Geir Bjørklund's interest and research also include medical anthropology,[83] nutrition and epigenetics,[84] urology,[85] and reproductive health.[86]
Council for Nutritional and Environmental Medicine
Bjørklund is founder and president of the Council for Nutritional and Environmental Medicine (CONEM).[2] CONEM is a non-profit association founded and registered in Norway in 2013. The organization is established to facilitate research and information activities related to nutritional and environmental medicine. The fundamental mission is to promote the health of the public at large. CONEM is creating a network of clinicians and researchers from different countries that want to collaborate. Also, the association creating working groups with people in different fields. CONEM has members in more than 60 countries. Members of the association are mainly dental, medical, and research professionals.[2]
At present (August 2017), the following CONEM groups exist: CONEM Germany Environmental Health and Safety Research Group, CONEM Poland Chemistry and Nutrition Research Group, CONEM Romania Biotechnology and Environmental Sciences Group, CONEM Ukraine Life Science Research Group, CONEM Kazakhstan Environmental Health and Safety Research Group, CONEM Jordan Environmental Health Research Group, CONEM Int. Cancer and Nutrition Research Group, CONEM Egypt Child Brain Research Group, CONEM Upper Egypt Pediatric Research Group, and CONEM US Autism Research Group.[2] The majority of the groups are based at universities.
In some countries, CONEM collaborates with other organizations, i.e. Swedish Society of Orthomolecular Medicine and Société Algérienne de Nutrition et de Médecine Orthomoléculaire (The Algerian Branch of CONEM, SANMO).[2]
Journals
Bjørklund is founder and was the first editor of Tenner & Helse, and the Nordic Journal of Biological Medicine (Nordisk Tidsskrift for Biologisk Medisin). He has also been freelance journalist for Sunnhetsbladet (see Early Career). Today, Geir Bjørklund is a member of the editorial boards of numerous international scientific journals. He is also a reviewer of manuscripts for about 20 other peer-reviewed journals, including journals in the fields neurosciences, nutrition, biochemistry, environmental chemistry, and toxicology.[2]
Awards and support
In 2003, the Albert Lindsay von Julin Foundation in Helsinki awarded Geir Bjørklund a scholarship of 10,000 Euros for his work to promote the understanding of biological medicine.[2] He has also received support from, among others, OSO Eco Foundation and Rana Utviklingsselskap AS (the development company of the municipality of Rana, Norway).[2] Bjørklund has been identified as being among the top 2.5% of all scientists worldwide by ResearchGate.[2]
References
- ↑ . Marquis Who's Who Archived 2012-05-24 at Archive.is
- 1 2 3 4 5 6 7 8 9 10 11 Council for Nutritional and Environmental Medicine
- ↑ Kennedy RF Jr. The interwoven global epidemics of mercury toxicity and autism. World Mercury Project 24.10.2017. https://worldmercuryproject.org/news/interwoven-global-epidemics-mercury-toxicity-autism (30.11.2017).
- ↑ Articles by Geir Bjørklund in Sunnhetsbladet. Norart.no
- ↑ Bjørklund G. The body’s chemical laboratory: the liver (in Norwegian). Sunnhetsbladet 1995, No. 12: 10-11.
- ↑ Bruk av tannrestaureringsmaterialer i Norge. Rapport nr. IK-2652. Oslo: Statens helsetilsyn, 1998.
- ↑ The use of dental filling materials in Norway. Archived 2011-09-28 at the Wayback Machine. Report No. IK-2675. Oslo: Norwegian Board of Health, 1999.
- ↑ Bjørklund G. Ny giv for biologisk medisin i Norden. Nord Tidsskr Biol Med 2001; 1: 3-6.
- ↑ Christensen B. Ambisiøst og uklart om biologisk medisin. Tidsskr Nor Lægeforen 2001; 121:3096.
- ↑ Bjørklund G. The history of dental amalgam (in Norwegian). Tidsskr Nor Laegeforen 1989; 109: 3582-3585.
- ↑ Bjørklund G. The first amalgam war: historical debate (in Norwegian). Sunnhetsbladet 1993, No. 11: 10-11.
- ↑ Bjørklund G. The second amalgam war: the historical debate continues (in Norwegian). Sunnhetsbladet 1993, nr 12: 10-11, 26.
- ↑ Bjørklund G. Health authorities and copper amalgam (in Norwegian). Tenner & Helse 1995, No. 2/3: 3-6.
- ↑ Bjørklund G. The history of dental amalgam (in Norwegian). Tenner & Helse 1997, No. 1: 13-20.
- ↑ Bjørklund G. Henrik Lichtenberg: A Tireless Proponent of Mercury-Free Dentistry (in Norwegian). Tenner & Helse 1998; No. 1-2-3: 18-27.
- ↑ Mijoč V. Dentalni amalgami danas – jesu li opasni? Split: Udruga Intender-hr, 2016. www.intender-hr.com/dentalni-amalgami-danas-jesu-li-opasni (10.02.2018)
- ↑ Bjørklund G. Dental amalgam – a threat to our health? (in Norwegian). Teknisk Ukeblad 1992; No. 33: 5 (correction in No. 35: 43).
- ↑ Pleva J, Bjørklund G. Mercury exposure from dental amalgam: a material question (in Norwegian). Tenner & Helse 1995, No. 1: 3-12.
- 1 2 3 Kern JK, Geier DA, Bjørklund G, King PG, Homme KG, Haley BE, Sykes LK, Geier MR. Evidence supporting a link between dental amalgams and chronic illness, fatigue, depression, anxiety, and suicide. Neuro Endocrinol Lett 2014; 35: 537-552.
- 1 2 Kvikksølv-plomber rammer tannlegen. Østlandets Blad 25.03.1991.
- 1 2 Kvikksølv helsefarlig. Adresseavisen 25.03.1991.
- ↑ Myhr KI. Ungt offer ble amalgam-ekspert. Dagbladet 03.04.1991: 14.
- 1 2 Bisseberg A. Kvikksølv årsak til senil demens? Aftenposten Aften 21.08.1991: 20.
- ↑ Hegge PE. Tvetydig nytt om amalgamfyllinger. Aftenposten Aften 16.09.1991: 5.
- 1 2 Bjørklund G. Mercury in the dental office. Risk evaluation of the occupational environment in dental care (in Norwegian). Tidsskr Nor Laegeforen 1991; 111: 948-951.
- ↑ Hamre HJ. Amalgam og sykdom. Oslo: Vidarforlaget, 1993.
- ↑ Myhr KI. Farlig for tannlegene. Dagbladet 03.04.1991.
- ↑ Bjørklund G. Mercury as a potential source for the etiology of Alzheimer’s disease. Trace Elements in Medicine 1991; 8: 208.
- ↑ Bjørklund G. Can mercury cause Alzheimer's disease? (in Norwegian). Tidsskr Nor Laegeforen 1991; 111: 2462.
- ↑ Hamilton K. Alzheimer's Disease: Mercury. CP Currents 1992; 2(3): 60.
- ↑ Bjørklund G. Parkinson disease, mercury and other heavy metals (in Norwegian). Tidsskr Nor Laegeforen 1995; 115: 757.
- ↑ Bjørklund G. Parkinson’s disease and mercury. Journal of Orthomolecular Medicine 1995; 10: 147-148.
- ↑ Bjørklund G. Morbus Parkinson und Quecksilber. Journal für Orthomolekulare Medizin 1996; 4: 235-237.
- ↑ Bjørklund G. Multiple sclerosis: a dental problem? (in Norwegian). Sunnhetsbladet 1991, nr. 12: 8-9.
- 1 2 3 4 5 6 7 8 9 10 11 Kern JK, Geier DA, Homme KG, King PG, Bjørklund G, Chirumbolo S, Geier MR. Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol Exp (Wars) 2017; 77: 269-296.
- ↑ Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, Bjørklund G, Skalnaya MG, Gatiatulina ER, Popova EV, Nemereshina ON, Vinceti M, Skalny AV. The role of cadmium in obesity and diabetes. Sci Total Environ 2017;601-602:741-755.
- ↑ Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjørklund G, Gatiatulina ER, Popova EV, Nemereshina ON, Huang PT, Vinceti M, Skalny AV. Cadmium and atherosclerosis: A review of toxicological mechanisms and a meta-analysis of epidemiologic studies. Environ Res 2018;162:240-260.
- ↑ Bjørklund G, Aaseth J, Chirumbolo S, Urbina MA, Uddin R. Effects of arsenic toxicity beyond epigenetic modifications. Environ Geochem Health 2017. doi: 10.1007/s10653-017-9967-9.
- ↑ Fakhri Y, Bjørklund G, Bandpei AM, Chirumbolo S, Keramati H, Hosseini Pouya R, Asadi A, Amanidaz N, Sarafraz M, Sheikhmohammad A, Alipour M, Baninameh Z, Mohseni SM, Sarkhosh M, Ghasemi SM. Concentrations of arsenic and lead in rice (Oryza sativa L.) in Iran: A systematic review and carcinogenic risk assessment. Food Chem Toxicol 2018; 113: 267-277.
- ↑ Bjørklund G, Christophersen OA, Chirumbolo S, Selinus O, Aaseth J. Recent aspects of uranium toxicology in medical geology. Environ Res. 2017 Jul;156:526-533.
- ↑ Markabayeva A, Bauer S, Pivina L, Bjørklund G, Chirumbolo S, Kerimkulova A, Semenova Y, Belikhina T. Increased prevalence of essential hypertension in areas previously exposed to fallout due to nuclear weapons testing at the Semipalatinsk Test Site, Kazakhstan. Environ Res 2018;167:129-135.
- 1 2 3 4 5 Bjørklund G. The Role of Zinc and Copper in Autism Spectrum Disorders. Acta Neurobiol Exp 2013; 73: 225–236.
- ↑ Bjørklund G. Psychiatry in light of the history (in Norwegian). Sunnhetsbladet 1992, No. 10: 14-15.
- ↑ Bjørklund G. Psychiatry investigated (in Norwegian). Sunnhetsbladet 1992, No. 11: 14-15, 28, 30.
- ↑ Alkhatib AJ, Bashir NA, Bjørklund G, Shotar AM, Rawashdeh WS, AlOmary M, Al-Kresha R. Depression among addictive patients in Jordan. Indian Research Journal of Pharmacy and Science 2014; 1, 3: 10-18.
- ↑ Bjørklund G. The immune system: Stress thoughts become sick bodies (in Norwegian). Sunnhetsbladet 1995, No. 4: 12-13.
- ↑ Bjørklund G. Stress and pain (in Norwegian). Sunnhetsbladet 1995, No. 10: 12-13.
- ↑ Bjørklund G. Psychoimmunology: the immune system and the neuroendocrine system (in Norwegian). Sunnhetsbladet 1995, No. 1: 12-12, 28.
- ↑ Bjørklund G. Psychoimmunology and stress (in Norwegian). Sunnhetsbladet 1995, No. 2: 14-15.
- ↑ Bjørklund G. Psychoimmunology and stress management (in Norwegian). Sunnhetsbladet 1995, No. 3: 18-19.
- 1 2 3 4 5 Saad K, Abdel-rahman AA, Elserogy YM, Al-Atram AA, Cannell JJ, Bjørklund G et al. Vitamin D Status in Autism Spectrum Disorders and the Efficacy of Vitamin D Supplementation in Autistic Children. Nutr Neurosci. Article first published online: 15 Apr 2015. doi:10.1179/1476830515Y.0000000019
- ↑ Bjørklund G. Barn med Aspergers syndrom. Tidsskr Nor Laegeforen 1998; 118: 1567-1569.
- ↑ Bjørklund G. Asperger syndrome: a literature study. Poster presented at the conference "Evidence Based Assessment and Intervention in Autism and Comorbid Disorders". Cluj-Napoca: Universitatea Babes-Bolyai, November 12–14, 2009.
- ↑ Endreffy I, Bjørklund G, Dicső F, Urbina MA, Endreffy E. Acid glycosaminoglycan (aGAG) excretion is increased in children with autism spectrum disorder. Metab Brain Dis. Article first published online: 14 Oct 2015.
- ↑ Skalny AV, Skalnaya MG, Bjørklund G, Nikonorov AA, Tinkov AA. Mercury as a possible link between maternal obesity and autism spectrum disorder. Med Hypotheses 2016;91:90-94. doi: 10.1016/j.mehy.2016.04.021.
- 1 2 Skalny AV, Simashkova NV, Klyushnik TP, Grabeklis AR, Bjørklund G, Skalnaya MG, Nikonorov AA, Tinkov AA. Hair toxic and essential trace elements in children with autism spectrum disorder. Metab Brain Dis 2017; 32: 195-202.
- 1 2 3 Crăciun EC, Ursu M, Predescu E, Bjørklund G, Dronca M. The status of whole blood zinc and copper levels in autistic children (abstract). Revista Română de Medicină de Laborator 2009; 15 (Suppl): 132-133.
- 1 2 3 4 Li SO, Wang JL, Bjørklund G, Zhao WN, Yin CH. Serum copper and zinc levels in individuals with autism spectrum disorders. Neuroreport 2014; 25: 1216-1220. doi: 10.1097/WNR.0000000000000251.
- 1 2 3 4 Macedoni-Lukšič M, Gosar D, Bjørklund G, Oražem J, Kodrič J, Lešnik-Musek P, Zupančič M, France-Štiglic A, Sešek-Briški A, Neubauer D, Osredkar J. Levels of metals in the blood and specific porphyrins in the urine in children with autism spectrum disorders. Biol Trace Elem Res. 2014 Sep 19. [Epub ahead of print]. doi:10.1007/s12011-014-0121-6
- ↑ Formae Mentis - Associazione Onlus. Il ruolo dello zinco e del rame nei disturbi dello spettro autistico. www.formaementis.it/index.php/autismo/9-autismo/29-il-ruolo-dello-zinco-e-del-rame-nei-disturbi-dello-spettro-autistico.html (22.10.2014).
- ↑ Skalny AV, Simashkova NV, Skalnaya AA, Klyushnik TP, Bjørklund G, Skalnaya MG, Tinkov AA. Assessment of gender and age effects on serum and hair trace element levels in children with autism spectrum disorder. Metab Brain Dis 2017; 32: 1675-1684.
- ↑ El-Ansary A, Bjørklund G, Tinkov AA, Skalny AV, Al Dera H. Relationship between selenium, lead, and mercury in red blood cells of Saudi autistic children. Metab Brain Dis 2017; 32: 1073-1080.
- ↑ Meguid NA, Anwar M, Bjørklund G, Hashish A, Chirumbolo S, Hemimi M, Sultan E. Dietary adequacy of Egyptian children with autism spectrum disorder compared to healthy developing children. Metab Brain Dis 2017; 32: 607-615.
- ↑ Kałużna-Czaplińska J, Jóźwik-Pruska J, Chirumbolo S, Bjørklund G. Tryptophan status in autism spectrum disorder and the influence of supplementation on its level. Metab Brain Dis 2017; 32: 1585-1593.
- 1 2 Saad K, Elserogy Y, Abdel Rahman AA, Al-Atram AA, Mohamad IL, ElMelegy TTH, Bjørklund G, El-Houfy AA. ADHD, Autism and Neuroradiological Complications among Phenylketonuric Children in Upper Egypt. Acta Neurol Belg. Article first published online: 10 JAN 2015. doi:10.1007/s13760-014-0422-8
- 1 2 3 4 Saad K, Eltayeb AA, Mohamad IL, Al-Atram AA, Elserogy Y, Bjørklund G, El-Houfey AA, Nicholson B. A randomized, placebo-controlled trial of digestive enzymes in children with autism spectrum disorders. Clin Psychopharmacol Neurosci 2015; 13(2):188-193.
- ↑ Tinkov AA, Filippini T, Ajsuvakova OP, Aaseth J, Gluhcheva YG, Ivanova JM, Bjørklund G, Skalnaya MG, Gatiatulina ER, Popova EV, Nemereshina ON, Vinceti M, Skalny AV. The role of cadmium in obesity and diabetes. Sci Total Environ 2017; 601-602: 741-755.
- ↑ Tinkov AA, Bjørklund G, Skalny AV, Holmgren A, Skalnaya MG, Chirumbolo S, Aaseth J. The role of the thioredoxin/thioredoxin reductase system in the metabolic syndrome: towards a possible prognostic marker? Cell Mol Life Sci 2018; 75: 1567-1586.
- 1 2 3 Bjørklund G, Dadar M, Aaseth J. Delayed-type hypersensitivity to metals in connective tissue diseases and fibromyalgia. Environ Res 2018; 161: 573-579.
- ↑ Stejskal V, Reynolds T, Bjørklund G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J Trace Elem Med Biol 2015; 31: 230-236.
- 1 2 3 Bjørklund G, Dadar M, Chirumbolo S, Aaseth J. Fibromyalgia and nutrition: Therapeutic possibilities? Biomed Pharmacother 2018; 103: 531-538.
- ↑ Stejskal V, Öckert K, Bjørklund G. Metal-induced inflammation triggers fibromyalgia in metal-allergic patients. Neuro Endocrinol Lett 2013; 34: 559-565.
- 1 2 Endreffy I, Bjørklund G, Szerafin L, Urbina MA, Chirumbolo S, Endreffy E. Plasma alpha-L-fucosidase activity in chronic inflammation and autoimmune disorders in a pediatric cohort of hospitalized patients. Immunologic Research 2017. doi: 10.1007/s12026-017-8943-x.
- ↑ Bjørklund G. Mercury: physical and chemical properties (in Norwegian). Tenner & Helse 1995, No. 1: 17-18.
- 1 2 Bjørklund G. Bruce Phillip Kyle: American physician with a private clinic in Aarhus (in Norwegian). Tenner & Helse 1999; No. 2: 6-16.
- 1 2 3 4 5 6 7 8 9 Bjørklund G. Clinical use of the metal chelators calcium disodium edetate, DMPS, and DMSA. Saudi J Kidney Dis Transpl 2015; 26: 611-612.
- 1 2 3 Bjørklund G. Selenium as an antidote in the treatment of mercury intoxication. BioMetals 2015; 28(4): 605-614.
- ↑ Bjørklund G, Chartrand M. Nutritional and environmental influences on autism spectrum disorder. J Nutr Disorders The 2016; 6(1): e123.
- ↑ Bjørklund G, Aaseth J, Ajsuvakova OP, Nikonorov AA, Skalny AV, Skalnaya MG, Tinkov AA. Molecular Interaction between mercury and selenium in neurotoxicity. Coord Chem Rev 2017; 332: 30-37.
- 1 2 Bjørklund G. The Adjuvant Nutritional Intervention in Cancer (ANICA) Trial. Nutr Cancer 2015;67:1355-1358.
- ↑ Coenzyme Q10 and selenium and breast cancer. www.q10facts.com/coenzyme-q10-and-selenium-and-breast-cancer (assessed 15 October 2017).
- ↑ Bjørklund G, Rajib SA, Saffoon N, Pen JJ, Chirumbolo S. Insights on melatonin as a major pharmacological target in cancer prevention: What’s new? Curr Med Chem 2018.
- ↑ Bjørklund G, Wilson DW, De Meester F, Takahashi T, Wilczynska A, Singh RB. Diet and Atherosclerosis in Ancient Periods. World Heart J 2015; 7(1): 51-61.
- ↑ Jalili M, Pati S, Rath B, Bjørklund G, Singh RB. Effect of Diet and Nutrients on Molecular Mechanism of Gene Expression Mediated by Nuclear Receptor and Epigenetic Modulation. Open Nutra J 2013; 6: 27-34.
- ↑ Bjørklund G. Prostatitis (in Norwegian). Sunnhetsbladet 1993, nr 1: 20, 30.
- ↑ Alkhatib AJ, Hamadneh J, Bjørklund G, Alrasheidi SA. The Role of Heat Shock Protein 90 in Human Reproduction. Res J Biol Sci 2014; 9: 161-164.
External links
- Biography on CONEM's website
- Council for Nutritional and Environmental Medicine (CONEM) is an international non-profit association based in Norway. CONEM is established by Geir Bjørklund to facilitate research and information activities related to nutritional and environmental medicine.
- Profile for Geir Bjørklund on ResearchGate Scientific papers.