GATM (gene)
Glycine amidinotransferase, mitochondrial is an enzyme that in humans is encoded by the GATM gene.[4][5]
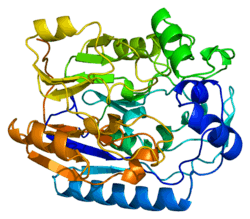
This gene encodes a mitochondrial enzyme that belongs to the Amidinotransferase family. This enzyme is involved in creatine biosynthesis, whereby it catalyzes the transfer of a guanido group from L-arginine to glycine, resulting in guanidinoacetic acid, the immediate precursor of creatine. Mutations in this gene cause arginine:glycine amidinotransferase deficiency, an inborn error of creatine synthesis characterized by mental retardation, language impairment, and behavioral disorders.[5]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000171766 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Humm A, Huber R, Mann K (Mar 1994). "The amino acid sequences of human and pig L-arginine:glycine amidinotransferase". FEBS Lett. 339 (1–2): 101–7. doi:10.1016/0014-5793(94)80394-3. PMID 8313955.
- 1 2 "Entrez Gene: GATM glycine amidinotransferase (L-arginine:glycine amidinotransferase)".
Further reading
- Humm A, Fritsche E, Steinbacher S (1997). "Structure and reaction mechanism of L-arginine:glycine amidinotransferase". Biol. Chem. 378 (3–4): 193–7. doi:10.1515/bchm.1997.378.3-4.121. PMID 9165070.
- Schulze A (2003). "Creatine deficiency syndromes". Mol. Cell. Biochem. 244 (1–2): 143–50. doi:10.1023/A:1022443503883. PMID 12701824.
- Gross MD, Eggen MA, Simon AM, Van Pilsum JF (1987). "The purification and characterization of human kidney L-arginine:glycine amidinotransferase". Arch. Biochem. Biophys. 251 (2): 747–55. doi:10.1016/0003-9861(86)90385-1. PMID 3800397.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Humm A; Fritsche E; Mann K; et al. (1997). "Recombinant expression and isolation of human L-arginine:glycine amidinotransferase and identification of its active-site cysteine residue". Biochem. J. 322 (3): 771–6. PMC 1218254. PMID 9148748.
- Humm A, Fritsche E, Steinbacher S, Huber R (1997). "Crystal structure and mechanism of human L-arginine:glycine amidinotransferase: a mitochondrial enzyme involved in creatine biosynthesis". EMBO J. 16 (12): 3373–85. doi:10.1093/emboj/16.12.3373. PMC 1169963. PMID 9218780.
- Fritsche E, Humm A, Huber R (1997). "Substrate binding and catalysis by L-arginine:glycine amidinotransferase--a mutagenesis and crystallographic study". Eur. J. Biochem. 247 (2): 483–90. doi:10.1111/j.1432-1033.1997.00483.x. PMID 9266688.
- Suzuki Y; Yoshirtomo-Nakagawa K; Maruyama K; et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Fritsche E, Humm A, Huber R (1999). "The ligand-induced structural changes of human L-Arginine:Glycine amidinotransferase. A mutational and crystallographic study". J. Biol. Chem. 274 (5): 3026–32. doi:10.1074/jbc.274.5.3026. PMID 9915841.
- Item CB; Stöckler-Ipsiroglu S; Stromberger C; et al. (2001). "Arginine:glycine amidinotransferase deficiency: the third inborn error of creatine metabolism in humans". Am. J. Hum. Genet. 69 (5): 1127–33. doi:10.1086/323765. PMC 1274356. PMID 11555793.
- Carducci C; Birarelli M; Leuzzi V; et al. (2002). "Guanidinoacetate and creatine plus creatinine assessment in physiologic fluids: an effective diagnostic tool for the biochemical diagnosis of arginine:glycine amidinotransferase and guanidinoacetate methyltransferase deficiencies". Clin. Chem. 48 (10): 1772–8. PMID 12324495.
- Battini R; Leuzzi V; Carducci C; et al. (2003). "Creatine depletion in a new case with AGAT deficiency: clinical and genetic study in a large pedigree". Mol. Genet. Metab. 77 (4): 326–31. doi:10.1016/S1096-7192(02)00175-0. PMID 12468279.
- Strausberg RL; Feingold EA; Grouse LH; et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Verhoeven NM; Schor DS; Roos B; et al. (2003). "Diagnostic enzyme assay that uses stable-isotope-labeled substrates to detect L-arginine:glycine amidinotransferase deficiency". Clin. Chem. 49 (5): 803–5. doi:10.1373/49.5.803. PMID 12709373.
- Ota T; Suzuki Y; Nishikawa T; et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Gerhard DS; Wagner L; Feingold EA; et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Alessandrì MG; Celati L; Battini R; et al. (2005). "Gas chromatography/mass spectrometry assay for arginine: glycine-amidinotransferase deficiency". Anal. Biochem. 343 (2): 356–8. doi:10.1016/j.ab.2005.05.003. PMID 15978539.
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.