Catalytic cycle
In chemistry, a catalytic cycle is a multistep reaction mechanism that involves a catalyst.[1] The catalytic cycle is the main method for describing the role of catalysts in biochemistry, organometallic chemistry, bioinorganic chemistry, materials science, etc. Often such cycles show the conversion of a precatalyst to the catalyst. Since catalysts are regenerated, catalytic cycles are usually written as a sequence of chemical reactions in the form of a loop. In such loops, the initial step entails binding of one or more reactants by the catalyst, and the final step is the release of the product and regeneration of the catalyst. Articles on the Monsanto process, the Wacker process, and the Heck reaction show catalytic cycles.
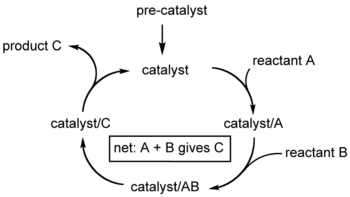
A catalytic cycle is not necessarily a full reaction mechanism. For example, it may be that the intermediates have been detected, but it is not known by which mechanisms the actual elementary reactions occur.
Catalytic cycles also have an important role in the atmosphere, for example in the reactions that lead to ozone depletion. The complicated interplay between different schemes, including null cycles that have no overall effect, determines the rate of production and destruction of many atmospheric components.
Sacrificial catalysts
Often a so-called sacrificial catalyst is also part of the reaction system with the purpose of regenerating the true catalyst in each cycle. As the name implies, the sacrificial catalyst is not regenerated and is irreversibly consumed, thereby not a catalyst at all. This sacrificial compound is also known as a stoichiometric catalyst when added in stoichiometric quantities compared to the main reactant. Usually the true catalyst is an expensive and complex molecule and added in quantities as small as possible. The stoichiometric catalyst on the other hand should be cheap and abundant. "Sacrificial catalysts" are more accurately referred to by their actual role in the catalytic cycle, for example as a reductant, a terminal oxidant, or an initiator.
References
- ↑ Kinetics of catalytic reactions 2005 M. Albert Vannice