FAHD1
Fumarylacetoacetate hydrolase domain-containing protein 1, also known as FLJ36880 protein, is an enzyme that in humans is encoded by the FAHD1 gene on chromosome 16.[5]
Structure
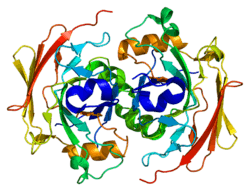
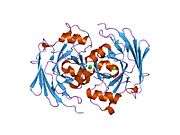
The FAHD1 gene encodes for a 24-kDa protein that is localized to the mitochondrion and belongs to the fumarylacetoacetate hydrolase family of proteins.[6] The structure of FAHD1 has been resolved using X-ray crystallography at 2.2-Å resolution. The overall structure is similar to the C-terminal domain of the bifunctional enzyme HpcE from Escherichia coli C, fumarylacetoacetate hydrolase from Mus musculus and to YcgM (Apc5008) from E. coli 1262.[6] A number of conserved amino acids including Asp-102 and Arg-106 of FAHD1 appear to be important for its catalytic activity.[7]
Function
The FAHD1 protein has been shown to function as an oxaloacetate decarboxylase in eukaryotes.[8] The FAHD1 protein probably also functions as an acylpyruvase, having been shown to catalyze the hydrolysis of acetylpyruvate and fumarylpyruvate in in vitro experiments.[6] Mg(2+) was required for maximal enzyme activity.[7]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000180185 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000045316 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: FAHD1 fumarylacetoacetate hydrolase domain containing 1".
- 1 2 3 Manjasetty BA, Niesen FH, Delbrück H, Götz F, Sievert V, Büssow K, Behlke J, Heinemann U (2004). "X-ray structure of fumarylacetoacetate hydrolase family member Homo sapiens FLJ36880". Biol. Chem. 385 (10): 935–42. doi:10.1515/BC.2004.122. PMID 15551868.
- 1 2 Pircher H, Straganz GD, Ehehalt D, Morrow G, Tanguay RM, Jansen-Dürr P (2011). "Identification of human fumarylacetoacetate hydrolase domain-containing protein 1 (FAHD1) as a novel mitochondrial acylpyruvase". J. Biol. Chem. 286 (42): 36500–8. doi:10.1074/jbc.M111.264770. PMC 3196145. PMID 21878618.
- ↑ Pircher H, von Grafenstein S, Diener T, Metzger C, Albertini E, Taferner A, Unterluggauer H, Kramer C, Liedl KR, Jansen-Dürr P (2015). "Identification of FAH domain-containing protein 1 (FAHD1) as oxaloacetate decarboxylase". J. Biol. Chem. 290 (11): 6755–62. doi:10.1074/jbc.M114.609305. PMC 4358102. PMID 25575590.
Further reading
- Hartley JL, Temple GF, Brasch MA (2001). "DNA cloning using in vitro site-specific recombination". Genome Res. 10 (11): 1788–95. doi:10.1101/gr.143000. PMC 310948. PMID 11076863.
- Daniels RJ, Peden JF, Lloyd C, et al. (2001). "Sequence, structure and pathology of the fully annotated terminal 2 Mb of the short arm of human chromosome 16". Hum. Mol. Genet. 10 (4): 339–52. doi:10.1093/hmg/10.4.339. PMID 11157797.
- Wiemann S, Weil B, Wellenreuther R, et al. (2001). "Toward a catalog of human genes and proteins: sequencing and analysis of 500 novel complete protein coding human cDNAs". Genome Res. 11 (3): 422–35. doi:10.1101/gr.GR1547R. PMC 311072. PMID 11230166.
- Simpson JC, Wellenreuther R, Poustka A, et al. (2001). "Systematic subcellular localization of novel proteins identified by large-scale cDNA sequencing". EMBO Rep. 1 (3): 287–92. doi:10.1093/embo-reports/kvd058. PMC 1083732. PMID 11256614.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Lehner B, Sanderson CM (2004). "A protein interaction framework for human mRNA degradation". Genome Res. 14 (7): 1315–23. doi:10.1101/gr.2122004. PMC 442147. PMID 15231747.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Wiemann S, Arlt D, Huber W, et al. (2004). "From ORFeome to biology: a functional genomics pipeline". Genome Res. 14 (10B): 2136–44. doi:10.1101/gr.2576704. PMC 528930. PMID 15489336.
- Manjasetty BA, Niesen FH, Delbrück H, et al. (2005). "X-ray structure of fumarylacetoacetate hydrolase family member Homo sapiens FLJ36880". Biol. Chem. 385 (10): 935–42. doi:10.1515/BC.2004.122. PMID 15551868.
- Mehrle A, Rosenfelder H, Schupp I, et al. (2006). "The LIFEdb database in 2006". Nucleic Acids Res. 34 (Database issue): D415–8. doi:10.1093/nar/gkj139. PMC 1347501. PMID 16381901.