Dimethyl fumarate
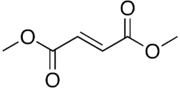 | |
| Names | |
|---|---|
| IUPAC name
Dimethyl (E)-butenedioate | |
| Other names
trans-1,2-Ethylenedicarboxylic acid dimethyl ester (E)-2-Butenedioic acid dimethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.009.863 |
| EC Number | 210-849-0 |
| UNII | |
| |
| |
| Properties | |
| C6H8O4 | |
| Molar mass | 144.13 g·mol−1 |
| Appearance | White crystalline solid |
| Density | 1.37 g/cm3 |
| Melting point | 103.5 °C (218.3 °F; 376.6 K)[1] |
| Boiling point | 193 °C (379 °F; 466 K)[1] |
| Pharmacology | |
| N07XX09 (WHO) | |
| License data | |
| Hazards | |
EU classification (DSD) (outdated) |
Harmful (Xn) |
| R-phrases (outdated) | R21 R38 R41 R43 |
| S-phrases (outdated) | S26 S36 S37 S39 |
| Related compounds | |
Related diesters |
Diethyl fumarate, dimethyl maleate, dimethyl malonate, dimethyl adipate |
Related compounds |
Fumaric acid Methyl acrylate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dimethyl fumarate (DMF) is the methyl ester of fumaric acid. DMF combined with three other fumaric acid esters (FAEs) was licensed in Germany as oral therapy for psoriasis (trade name Fumaderm).[2] Since 2013 it is used to treat adults with relapsing multiple sclerosis (trade name Tecfidera).[3] DMF is thought to have immunomodulatory properties without significant immunosuppression.[4]
In a non-medical use, DMF was applied as a biocide in furniture or shoes to prevent growths of mold during storage or transport in a humid climate. However, due to incidences of allergic reactions after skin contact the European Union has banned DMF in consumer products since 1998, and since January 2009 the import of products containing DMF has also been banned.[5]
Medical use
Dimethyl fumarate is used to treat psoriasis in an oral formulation mixed with related compounds[2] as well as in pure oral formulations.[6] It is also used in oral formulations to treat adults with relapsing multiple sclerosis.[3]
There is no information on how DMF affects the fetus during pregnancy; in animal tests there was fetal harm at clinically relevant doses.[3]
Dimethyl fumarate has also been developed as a heme oxygenase inducer conjugated to carbon monoxide-releasing molecules.
Adverse effects
The drug label includes warnings about the risk of anaphylaxis and angioedema, progressive multifocal leukoencephalopathy (PML), lymphopenia, and liver damage; other adverse effects include flushing and gastrointestinal events, such as diarrhea, nausea, and upper abdominal pain.[3][7]
Pharmacology
Dimethyl fumarate is a lipophilic, highly mobile molecule in human tissue. As an α,β-unsaturated electrophilic compound, DMF is rapidly attacked by the detoxifying agent glutathione (GSH) in a Michael addition reaction.[8][9][10] and through those reactions is metabolized to monomethyl fumarate (MMF) prior to entering systemic distribution; in clinical trials of Biogen's formulation DMF was not quantifiable in plasma following oral administration, and all pharmokinetic analysis was done measuring MMF.[3] DMF has been described a prodrug.[11]
The precise mechanism of action of dimethyl fumarate is unknown. DMF and MMF activate the transcription factor (erythroid-derived 2)-like 2 (Nrf2) pathway and has been identified as a nicotinic acid receptor agonist in vitro.[3]
Synthesis
Several methods exist for the laboratory synthesis of dimethyl fumarate, with reported methods alkene isomerization of dimethyl maleate,[12][13][14] and Fischer esterification of fumaric acid [12]
History
Dimethyl fumarate is an old compound used in industrial chemistry, and can be purchased by the ton; as of 2012 one could purchase it for $1 to $50 per metric ton, with a two-ton minimum purchase.[15][16]
Its first medical use was described in 1959 by a German physician, which was a topical formulation for psoriasis. The Swiss company Fumapharm eventually brought an oral formulation of DMF (along with some monoesters) to market for psoriasis in Germany in 1994.[16][17]
Some people using the cream for psoriasis also had MS, and their MS improved; this led to clinical studies for that purpose.[17] Fumapharm was one of them, and Biogen Idec collaborated with them on that work and then acquired Fumapharm in 2006.[16][18] Another company, Aditech Pharma in Sweden, had also been researching oral formulations of DMF for MS, and in 2010 the Danish company Forward Pharma acquired Aditech's patents.[18]
Meanwhile, Biogen continued developing its oral formulation of DMF from Fumapharm under the code name BG-12; it was approved, under the trade name Tecfidera, for the treatment of adults with relapsing forms of MS in March 2013.[19] Biogen priced the drug at $54,000 per year in the US.[16] It was approved in Europe in 2014.[20] In the UK NICE issued a guidance recommending the drug as cost-effective, but only if person does not have highly active or rapidly evolving severe relapsing–remitting multiple sclerosis and only if Biogen agreed to provide it at a discount.[21]
Forward and Biogen entered into patent litigation in many jurisdictions; in 2017 the companies settled the litigation, with Biogen paying Forward $1.25 billion, with the potential for up to 10% royalties depending on what happened with the patents in various jurisdictions.[18]
An oral formulation of DMF was approved in Europe for the treatment of plaque psoriasis in adults, under the brand name Skilarence by Amirall; it was approved in June 2017.[6]
Consumer products
Dimethyl fumarate has been found to be an allergic sensitizer at very low concentrations, producing eczema that is difficult to treat. Concentrations as low as 1 ppm may produce allergic reactions.[22] There are only a handful of equally potent sensitizers.[23]
The sensitizing risk was brought to public attention by the "poison chair" incident, where Chinese manufacturer Linkwise produced two-seater sofas with dimethyl fumarate sachets inside to inhibit mold while they were in storage or transport.[24] In Finland where the chairs were sold from 2006–2007, sixty users were given serious rashes.[23] The cause was identified as dimethyl fumarate-induced allergic reaction by Tapio Rantanen from Finland, and his original article became the cover story in the July issue of the British Journal of Dermatology.[22] In the United Kingdom, sofas sold by Argos, Land of Leather and Walmsley Furnishing containing the chemical caused over a hundred injuries.[23] Argos withdrew the sofas from stores and contacted buyers to collect those that had been sold — with Land of Leather withdrawing the sofas without notifying buyers and Walmsley saying they had removed the sachets from sofas they sold after the danger came to light.[25][26] The danger came to public attention in 2008 when the BBC Watchdog program alerted consumers to the sofas.[25][27]
In the European Union, the use of dimethyl fumarate for consumer products has been forbidden since 1998, and in January 2009 it was proposed that the import of consumer products containing dimethyl fumarate is also forbidden.[5]
The EU Commission Decision 2009/251 of 17 March 2009 requiring Member States to ensure that products containing the biocide dimethyl fumarate are not placed or made available on the market from 1 May 2009 has definitely forbidden any marketing of products containing dimethyl fumarate into the European Union.[28] The ban on dimethyl fumarate as laid down in Decision 2009/251 establishes a maximum concentration of dimethyl fumarate in products of 0.1 ppm. Products containing more than 0.1 ppm dimethyl fumarate shall be withdrawn from the market and recalled from consumers.
References
- 1 2 "Background document to the opinions on the Annex XV dossier proposing restrictions on Dimethylfumarate (DMFu)" (PDF). European Chemicals Agency. 16 March 2011. p. 9.
- 1 2 Mrowietz, Ulrich; Altmeyer, Peter; Bieber, Thomas; et al. (2007). "Treatment of psoriasis with fumaric acid esters (Fumaderm®)". JDDG. 5 (8): 716–7. doi:10.1111/j.1610-0387.2007.06346.x. PMID 17659047.
- 1 2 3 4 5 6 "Dimethyl fumarate label" (PDF). FDA. December 2017. Retrieved 19 July 2018. For label updates see FDA index page for NDA 204063
- ↑ McEntee, Joanne. "Fumaderm®: what is the evidence for its efficacy and safety". North West Medicines Information Centre. Archived from the original on 5 May 2013. Retrieved 29 November 2012.
- 1 2 "Consumers: EU to ban dimethylfumarate (DMF) in consumer products, such as sofas and shoes" (Press release). European Commission. Retrieved 29 November 2012.
- 1 2 "Skilarence 120 mg Gastro-resistant Tablets - Summary of Product Characteristics". Electronic Medicines Compendium. May 2018. Retrieved 19 July 2018.
- ↑ "FDA Drug Safety Communication: FDA warns about case of rare brain infection PML with MS drug Tecfidera (dimethyl fumarate)". fda.gov. 2014-11-25. Retrieved 2 December 2014.
- ↑ Boyland, E; Chasseaud, LF (1967). "Enzyme-catalysed conjugations of glutathione with unsaturated compounds". The Biochemical Journal. 104 (1): 95–102. doi:10.1042/bj1040095. PMC 1270549. PMID 6035529.
- ↑ Kubal, Gina; Meyer, David J.; Norman, Richard E.; Sadler, Peter J. (1995). "Investigations of Glutathione Conjugation in Vitro by 1H NMR Spectroscopy. Uncatalyzed and Glutathione Transferase-Catalyzed Reactions". Chemical Research in Toxicology. 8 (5): 780–91. doi:10.1021/tx00047a019. PMID 7548762.
- ↑ Schmidt, Thomas J.; Ak, Muharrem; Mrowietz, Ulrich (2007). "Reactivity of dimethyl fumarate and methylhydrogen fumarate towards glutathione and N-acetyl-l-cysteine—Preparation of S-substituted thiosuccinic acid esters". Bioorganic & Medicinal Chemistry. 15 (1): 333–42. doi:10.1016/j.bmc.2006.09.053. PMID 17049250.
- ↑ Goldhill, Jon (September 15, 2015). "Disappointing Phase 2 psoriasis data reported for the monomethyl fumarate prodrug, XP23829". Advances in drug discovery.
- 1 2 Two Approaches to the Synthesis of Dimethyl Fumarate That Demonstrate Fundamental Principles of Organic Chemistry Brian E. Love and Lisa J. Bennett Journal of Chemical Education 2017 94 (10), 1543-1546 doi:10.1021/acs.jchemed.6b00818
- ↑ Isomerization of dimethyl maleate to dimethyl fumarate: An undergraduate experiment utilizing high performance liquid chromatography David B. Ledlie, Thomas J. Wenzel, and Susan M. Hendrickson Journal of Chemical Education 1989 66 (9), 781 doi:10.1021/ed066p781
- ↑ Isomerization of dimethyl maleate to dimethyl fumarate: An undergraduate experiment illustrating amine-catalyzed alkene isomerization, stereochemical principles, sublimation, and product identification by spectroscopic methods Craig B. Fryhle, Carol M. Rybak, and Kenneth E. Pulley Journal of Chemical Education 1991 68 (12), 1050 doi:10.1021/ed068p1050
- ↑ Hamilton, Mike (13 September 2012). "Just how much profit is there in a new drug?". Biotech Translated.
- 1 2 3 4 Lowe, Derek (2 April 2013). "Tecfidera's Price". In the Pipeline.
- 1 2 Ettmayer, P; Schnitzer, R; Bergner, A; Nar, H (2017). "Chapter 2.02: Hit and Lead Generation Strategies". In Chackalamannil, Samuel; Rotella, David; Ward, Simon; Martinez, Ana; Gil, Carmen. Comprehensive Medicinal Chemistry III: Volume 2: Drug Discovery Technologies. Elsevier. p. 58. ISBN 9780128032015.
- 1 2 3 Vinluan, Frank (17 January 2017). "Xconomy: Biogen to Pay $1.25B to Settle Forward Pharma Patent Suit on MS Drug". Xconomy.
- ↑ "FDA approves new multiple sclerosis treatment: Tecfidera". FDA. Mar 27, 2013. Retrieved 2013-04-05.
- ↑ "Tecfidera 120mg and 240mg gastro-resistant hard capsules - Summary of Product Characteristics". Electronic Medicines Compendium. February 2018. Retrieved 19 July 2018.
- ↑ Burke, MJ; Richardson, J; George, E; Adler, AI (November 2014). "Dimethyl fumarate for relapsing-remitting multiple sclerosis". The Lancet. Neurology. 13 (11): 1077–1078. doi:10.1016/S1474-4422(14)70159-0. PMID 28845815.

- 1 2 Rantanen, T. (2008). "The cause of the Chinese sofa/chair dermatitis epidemic is likely to be contact allergy to dimethylfumarate, a novel potent contact sensitizer". British Journal of Dermatology. 159 (1): 218–21. doi:10.1111/j.1365-2133.2008.08622.x. PMID 18503603.
- 1 2 3 "Myrkkytuoli-ihottumien syy selvisi". YLE Uutiset (in Finnish). YLE. 2008-04-24. Retrieved 2008-06-10.
- ↑ "Clifton woman's toxic sofa claim". The Nottingham Post. Local World. 2 Apr 2009.
- 1 2 BBC - Consumer - TV and radio - itchy sofas Archived February 22, 2008, at the Wayback Machine.
- ↑ BBC: Judge rejects 'toxic sofa' claims in burns injury cases, 18 March 2010
- ↑ "BBC: 3 reports on dimethyl fumarate in furniture". April 2010.
- ↑ "2009/251/EC: Commission Decision of 17 March 2009".
External links
- fishersci.ca: Dimethyl fumarate Material Safety Data Sheet (MSDS)