Diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. The most popular derivatives are 1,2-diketones and 1,3-diimines. These compounds are used as ligands and as precursors to heterocycles. Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Similar methods are used to prepare Schiff bases and oximes.
1,2-Diimines
The 1,2-diketimine ligands, also called α-diimines, include dimethylglyoxime as well as oxidized derivatives of o-phenylenediamine. The steric properties of the substituents on nitrogen provide a means to control the axial coordination sites on a square planar complex. Large planar substituents such as mesityl tend to be orthogonal to the MN2 plane. In this way, the axial coordination sites on a square planar complex are shielded. Such steric control is not possible for complexes of the related to 2,2'-bipyridine, glyoximate, and 9,10-phenanthroline ligands.
An example is glyoxal-bis(mesitylimine), a yellow solid that is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal.[1]
1,2-Diketimines, but not the 1,3-diketimines, are “non-innocent ligands”, akin to the dithiolenes.
1,3-Diimines
For example, acetylacetone (2,4-pentanedione) and a primary alkyl- or arylamine will react, typically in acidified ethanol, to form a diketimine. 1,3-Diketimines are often referred to as HNacNac, a modification of the abbreviation Hacac for the conjugate acid of acetylacetone. These species form anionic bidentate anionic ligands.
Uses
Substituted α-diimine ligands are useful in the preparation of so-called post-metallocene catalysts for the polymerization and copolymerization of ethylene and alkenes.[2]
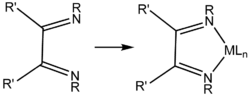
Diimines are precursors to NHC ligands by condensation with formaldehyde.[1]
References
- 1 2 Elon A. Ison, Ana Ison "Synthesis of Well-Defined Copper N-Heterocyclic Carbene Complexes and Their Use as Catalysts for a “Click Reaction”: A Multistep Experiment That Emphasizes the Role of Catalysis in Green Chemistry" J. Chem. Educ., 2012, volume 89, pp 1575–1577. doi:10.1021/ed300243s
- ↑ Ittel, S. D.; Johnson, L. K.; Brookhart, M. (2000). "Late-Metal Catalysts for Ethylene Homo- and Copolymerization". Chemical Reviews. 100: 1169–1203. doi:10.1021/cr9804644.