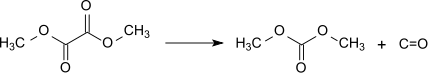Decarbonylation
Decarbonylation is a type of organic reaction that involves loss of CO. It is often an undesirable reaction since it represents a degradation. In the chemistry of metal carbonyls, decarbonylation describes a substitution process, whereby a CO ligand is replaced by another ligand.
Organic chemistry
In the absence of metal catalysts, decarbonylation (vs decarboxylation) is rarely observed in organic chemistry. One exception is the decarbonylation of formic acid:
- HCO2H → CO + H2O
The reaction is induced by sulfuric acid, which functions as both a catalyst and a dehydrating agent. Via this reaction, formic acid is occasionally employed as a source of CO in the laboratory in lieu of cylinders of this toxic gas.[1] With strong heating, formic acid and some of its derivatives may undergo decarbonylation, even without adding a catalyst. For instance, dimethylformamide slowly decomposes to give dimethylamine and carbon monoxide when heated to its boiling point (154 °C).
Silacarboxylic acids (R3SiCOOH) undergo decarbonylation upon heating or treatment with base and have been investigated as carbon monoxide generating molecules.[2][3]
Aldehyde decarbonylation
A common transformation involves the conversion of aldehydes to alkanes.[4] The reverse reaction, carbonylation, involves the insertion of CO into a bond, is a common and industrially relevant reaction.
Decarbonylation is catalyzed by soluble metal complexes:.[5][4]
- RCHO → RH + CO
These reactions proceed via the intermediacy of metal acyl hydrides. An example of this is the Tsuji-Wilkinson decarbonylation reaction
Decarbonylations are of interest in the conversions of sugars.[6] Ketones and other carbonyl-containing functional groups are more resistant to decarbonylation than are aldehydes.
Biochemistry
Carbon monoxide is released in the degradation (catabolism) of heme by the action of O2, NADPH and the enzyme heme oxygenase:[7]
- Heme b + 3 O2 + 3½NADPH + 3½H+ → biliverdin + Fe2+ + CO + 3½NADP+ + 3H2O
Inorganic and organometallic synthesis
Many metal carbonyls are prepared via decarbonylation reactions. The CO ligand in Vaska's complex arises by the decarbonylation of DMF:
- IrCl3(H2O)3 + 3 P(C6H5)3 + HCON(CH3)2 + C6H5NH2 → IrCl(CO)[P(C6H5)3]2 + [(CH3)2NH2]Cl + OP(C6H5)3 + [C6H5NH3]Cl + 2 H2O
The conversion of Fe(CO)5 and Mo(CO)6 to their many derivatives often involves decarbonylation. Here decarbonylation accompanies the preparation of Cyclopentadienyliron dicarbonyl dimer:
- 2 Fe(CO)5 + C10H12 → (η5-C5H5)2Fe2(CO)4 + 6 CO + H2
Decarbonylation can be induced photochemically as well as using reagents such as trimethylamine-N-oxide:
- Me3NO + L + Fe(CO)5 → Me3N + CO2 + LFe(CO)4
References
- ↑ Koch, H.; Haaf, W. (1973). "1-Adamantanecarboxylic Acid". Organic Syntheses. ; Collective Volume, 5, p. 20
- ↑ Brook, A. G.; Gilman, Henry (April 1955). "Base-catalyzed Elimination Reactions of Triphenylsilanecarboxylic Acid and its Derivatives". Journal of the American Chemical Society. 77 (8): 2322–2325. doi:10.1021/ja01613a088. ISSN 0002-7863.
- ↑ Friis, Stig D.; Taaning, Rolf H.; Lindhardt, Anders T.; Skrydstrup, Troels (2011-11-16). "Silacarboxylic Acids as Efficient Carbon Monoxide Releasing Molecules: Synthesis and Application in Palladium-Catalyzed Carbonylation Reactions". Journal of the American Chemical Society. 133 (45): 18114–18117. doi:10.1021/ja208652n. ISSN 0002-7863.
- 1 2 Kreis, M.; Palmelund, A.; Bunch, L.; Madsen, R., "A General and Convenient Method for the Rhodium-Catalyzed Decarbonylation of Aldehydes", Advanced Synthesis & Catalysis 2006, 348, 2148-2154. doi:10.1002/adsc.200600228
- ↑ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010.
- ↑ Geilen, F. M. A.; vom Stein, T.; Engendahl, B.; Winterle, S.; Liauw, M. A.; Klankermayer, J.; Leitner, W., "Highly Selective Decarbonylation of 5-(Hydroxymethyl)Furfural in the Presence of Compressed Carbon Dioxide", Angew. Chem. Int. Ed. 2011, 50, 6831-6834. doi:10.1002/anie.201007582
- ↑ Ryter, S. W.; Tyrrell, R. M., "The Heme Synthesis and Degradation Pathways: Role in Oxidant Sensitivity: Heme Oxygenase Has Both Pro- and Antioxidant Properties", Free Radical Biology and Medicine 2000, volume 28, pages 289-309. doi:10.1016/S0891-5849(99)00223-3