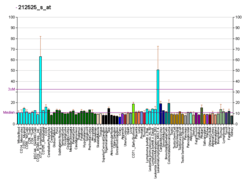
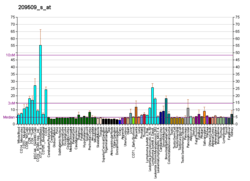
DPAGT1
UDP-N-acetylglucosamine—dolichyl-phosphate N-acetylglucosaminephosphotransferase is an enzyme that in humans is encoded by the DPAGT1 gene.[5][6]
Mutations in DPAGT1 cause myasthenia .Selcen, D; Shen, X. M.; Brengman, J; Li, Y; Stans, A. A.; Wieben, E; Engel, A. G. (2014). "DPAGT1 myasthenia and myopathy: Genetic, phenotypic, and expression studies". Neurology. 82 (20): 1822–30. doi:10.1212/WNL.0000000000000435. PMC 4035711. PMID 24759841.
The protein encoded by this gene is an enzyme that catalyzes the first step in the dolichol-linked oligosaccharide pathway (also see Genetic pathway) for glycoprotein biosynthesis. This enzyme belongs to the glycosyltransferase family 4. This protein is an integral membrane protein of the endoplasmic reticulum. The congenital disorder of glycosylation type Ij is caused by mutation in the gene encoding this enzyme. Alternatively spliced transcript variants encoding different isoforms have been identified.[6]
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000172269 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000032123 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Smith MW, Clark SP, Hutchinson JS, Wei YH, Churukian AC, Daniels LB, Diggle KL, Gen MW, Romo AJ, Lin Y, et al. (Dec 1993). "A sequence-tagged site map of human chromosome 11". Genomics. 17 (3): 699–725. doi:10.1006/geno.1993.1392. PMID 8244387.
- 1 2 "Entrez Gene: DPAGT1 dolichyl-phosphate (UDP-N-acetylglucosamine) N-acetylglucosaminephosphotransferase 1 (GlcNAc-1-P transferase)".
Further reading
- Freeze HH (2002). "Update and perspectives on congenital disorders of glycosylation". Glycobiology. 11 (12): 129R–143R. doi:10.1093/glycob/11.12.129R. PMID 11805072.
- Freeze HH (2003). "Human disorders in N-glycosylation and animal models". Biochim. Biophys. Acta. 1573 (3): 388–93. doi:10.1016/S0304-4165(02)00408-7. PMID 12417423.
- Miller BS, Freeze HH (2003). "New disorders in carbohydrate metabolism: congenital disorders of glycosylation and their impact on the endocrine system". Reviews in endocrine & metabolic disorders. 4 (1): 103–13. doi:10.1023/A:1021883605280. PMID 12618564.
- Volpe JJ, Sakakihara Y, Ishii S (1987). "Dolichol-linked glycoprotein synthesis in developing mammalian brain: maturational changes of the N-acetylglucosaminylphosphotransferase". Brain Res. 430 (2): 277–84. doi:10.1016/0165-3806(87)90160-x. PMID 3038274.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Bonaldo MF, Lennon G, Soares MB (1997). "Normalization and subtraction: two approaches to facilitate gene discovery". Genome Res. 6 (9): 791–806. doi:10.1101/gr.6.9.791. PMID 8889548.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Eckert V, Blank M, Mazhari-Tabrizi R, et al. (1998). "Cloning and functional expression of the human GlcNAc-1-P transferase, the enzyme for the committed step of the dolichol cycle, by heterologous complementation in Saccharomyces cerevisiae". Glycobiology. 8 (1): 77–85. doi:10.1093/glycob/8.1.77. PMID 9451016.
- Meissner JD, Naumann A, Mueller WH, Scheibe RJ (1999). "Regulation of UDP-N-acetylglucosamine:dolichyl-phosphate N-acetylglucosamine-1-phosphate transferase by retinoic acid in P19 cells". Biochem. J. 338 (2): 561–8. doi:10.1042/0264-6021:3380561. PMC 1220086. PMID 10024536.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Regis S, Dagnino F, Caroli F, Filocamo M (2003). "Genomic structure of the human UDP-GlcNAc:dolichol-P GlcNAc-1-P transferase gene". DNA Seq. 13 (5): 245–50. doi:10.1080/1042517021000017126. PMID 12592703.
- Newell JW, Seo NS, Enns GM, et al. (2004). "Congenital disorder of glycosylation Ic in patients of Indian origin". Mol. Genet. Metab. 79 (3): 221–8. doi:10.1016/S1096-7192(03)00089-1. PMID 12855228.
- Wu X, Rush JS, Karaoglu D, et al. (2004). "Deficiency of UDP-GlcNAc:Dolichol Phosphate N-Acetylglucosamine-1 Phosphate Transferase (DPAGT1) causes a novel congenital disorder of Glycosylation Type Ij". Hum. Mutat. 22 (2): 144–50. doi:10.1002/humu.10239. PMID 12872255.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
External links