DEET
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N-Diethyl-3-methylbenzamide | |
| Other names
N,N-Diethyl-m-toluamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.682 |
| KEGG | |
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C12H17NO | |
| Molar mass | 191.27 g/mol |
| Density | 0.998 g/mL |
| Melting point | −33 °C (−27 °F; 240 K) |
| Boiling point | 288 to 292 °C (550 to 558 °F; 561 to 565 K) |
| Pharmacology | |
| P03BX02 (WHO) QP53GX01 (WHO) | |
| Hazards | |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| GHS signal word | DANGER |
| H301, H304, H311, H331 | |
| NFPA 704 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
N,N-Diethyl-meta-toluamide, also called DEET (/diːt/) or diethyltoluamide, is the most common active ingredient in insect repellents. It is a slightly yellow oil intended to be applied to the skin or to clothing and provides protection against mosquitoes, ticks, fleas, chiggers, leeches and many biting insects.
History
DEET was developed in 1944[1] by Samuel Gertler[1] of the United States Department of Agriculture for use by the United States Army,[2] following its experience of jungle warfare during World War II. It was originally tested as a pesticide on farm fields, and entered military use in 1946 and civilian use in 1957. It was used in Vietnam and Southeast Asia.[3]
In its original form known as "bug juice", the application solution for DEET was composed of 75% DEET and ethanol.[4] Later, a new version of the repellent was developed by U.S. Army and the USDA. This incarnation consisted of DEET and polymers that extended the release of the DEET and reduced its evaporation rate.[4] This extended-release application was registered by the EPA in 1991.[4]
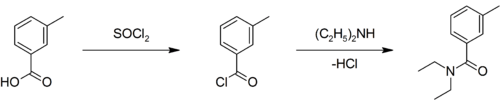
Preparation
A slightly yellow liquid at room temperature, it can be prepared by converting m-toluic acid (3-methylbenzoic acid) to the corresponding acyl chloride using thionyl chloride (SOCl2), and then allowing that product to react with diethylamine:[5][6]
Mechanism and effectiveness
DEET was historically believed to work by blocking insect olfactory receptors for 1-octen-3-ol, a volatile substance that is contained in human sweat and breath. The prevailing theory was that DEET effectively "blinds" the insect's senses so that the biting/feeding instinct is not triggered by humans or other animals which produce these chemicals. DEET does not appear to affect the insect's ability to smell carbon dioxide, as had been suspected earlier.[7][8]
However, more recent evidence shows that DEET serves as a true repellent in that mosquitoes intensely dislike the smell of the chemical.[9] A type of olfactory receptor neuron in special antennal sensilla of mosquitoes that is activated by DEET, as well as other known insect repellents such as eucalyptol, linalool, and thujone, has been identified. Moreover, in a behavioral test, DEET had a strong repellent activity in the absence of body odor attractants such as 1-octen-3-ol, lactic acid, or carbon dioxide. Both female and male mosquitoes showed the same response.[9][10]
A 2011 structural study (PDB: 3N7H) revealed that DEET binds to Anopheles gambiae odorant binding protein 1 (AgamOBP1) with high shape complementarity, suggesting that AgamOBP1 is a molecular target of DEET and perhaps other repellents.[11]
A 2013 study suggests that mosquitoes can at least temporarily overcome or adapt to the repellent effect of DEET after an initial exposure, representing a non-genetic behavioral change.[12] This observation, if verified, has significant implications for how repellent effectiveness should be assessed.
A highly conserved protein member of the ionotropic receptor family, IR40a, has recently been identified as a putative DEET receptor in the fly antenna. The neurons expressing IR40a respond to DEET in an IR40a dependent manner. Avoidance to DEET vapors is lost in flies that lack IR40a. Other repellent chemicals that are structurally related to DEET also activate the same receptor and repel mosquitoes and flies, suggesting that this receptor can be used to screen for new repellents.[13]
The hypothesis that IR40a is involved in DEET reception in mosquitoes has been tested in the southern house mosquito, Culex quinquefasciatus.[14] Reducing CquiIR40a transcript levels did not affect DEET-elicited behavior or electroantennographic (EAG) responses thus suggesting that IR40a is not involved in DEET reception in Culex mosquitoes. An antennae-specific odorant receptor, CquiOR136, was demonstrated to respond to DEET and other insect repellents. Similar knockout experiments showed that EAG responses to DEET recorded from mosquitoes with reduced CquiOR136 transcript levels were dramatically lower. Additionally, behavioral tests showed that this phenotype was not repelled by DEET. In conclusion, these results suggest that CquiOR136, not CquiIR40a, is involved in DEET reception in the southern house mosquito.[14]
Concentrations
Insect repellents containing DEET are the most effective. The concentration of DEET in products may range from less than 10 percent to over 30 percent. The benefits of DEET reach a peak at a concentration of 30 percent, the maximum concentration currently recommended for infants and children. DEET should not be used on children under 2 months of age.[15]
DEET is often sold and used in spray or lotion in concentrations up to 100%.[16] Consumer Reports found a direct correlation between DEET concentration and hours of protection against insect bites. 100% DEET was found to offer up to 12 hours of protection while several lower concentration DEET formulations (20–34%) offered 3–6 hours of protection.[17] Other research has corroborated the effectiveness of DEET.[18] The Centers for Disease Control and Prevention recommends 30–50% DEET to prevent the spread of pathogens carried by insects.[19]
Effects on health
As a precaution, manufacturers advise that DEET products should not be used under clothing or on damaged skin, and that preparations be washed off after they are no longer needed or between applications.[20] DEET can act as an irritant;[7] in rare cases, it may cause severe epidermal reactions.[20] Other symptoms that can occur are breathing difficulty, burning eyes, headaches.[21]
“DEET has a remarkable safety profile after 40 years of use and nearly 8 billion human applications. When applied with common sense, DEET-based repellents can be expected to provide a safe as well as long-lasting repellent effect. Despite the substantial attention paid by the lay press every year to the safety of DEET, this repellent has been subjected to more scientific and toxicological scrutiny than any other repellent substance."[22]
In the DEET Reregistration Eligibility Decision (RED) in 1998, the United States Environmental Protection Agency (EPA) reported 14 to 46 cases of potential DEET-associated seizures, including 4 deaths. The EPA states: "... it does appear that some cases are likely related to DEET toxicity," which may underreport the risk as physicians may fail to check for history of DEET use or fail to report cases of seizure subsequent to DEET use.[23]
The Pesticide Information Project of Cooperative Extension Offices of Cornell University states that "Everglades National Park employees having extensive DEET exposure were more likely to have insomnia, mood disturbances and impaired cognitive function than were lesser exposed co-workers".[24]
When used as directed, products containing between 10% and 30% DEET have been found by the American Academy of Pediatrics to be safe to use on children, as well as adults, but recommends that DEET not be used on infants less than two months old.[20]
Citing human health reasons, Health Canada barred the sale of insect repellents for human use that contained more than 30% DEET in a 2002 re-evaluation. The agency recommended that DEET-based products be used on children between the ages of 2 and 12 only if the concentration of DEET is 10% or less and that repellents be applied no more than 3 times a day, children under 2 should not receive more than 1 application of repellent in a day and DEET-based products of any concentration should not be used on infants under 6 months.[25][26] Some experts recommend against applying DEET and sunscreen simultaneously since that would increase DEET penetration; Canadian researcher, Xiaochen Gu, a professor at the University of Manitoba’s faculty of Pharmacy who led a study about mosquitos, advises that DEET should be applied 30 or more minutes later. Gu also recommends DEET sprays instead of lotions which are rubbed into the skin "forcing molecules into the skin".[27]
DEET is commonly used in combination with insecticides and can strengthen the toxicity of carbamate insecticides,[28] which are also acetylcholinesterase inhibitors. These findings indicate that DEET has neurological effects on insects in addition to known olfactory effects, and that its toxicity is strengthened in combination with other insecticides.
Detection in body fluids
DEET may be quantitated in blood, plasma or urine by gas or liquid chromatography-mass spectrometry to confirm a diagnosis of poisoning in hospitalized patients or to provide evidence in a medicolegal death investigation. Blood or plasma DEET concentrations are expected to be in a range of 0.3-3.0 mg/L during the first 8 hours after dermal application in persons using the chemical appropriately, >6 mg/L in intoxicated patients and >100 mg/L in victims of acute intentional oral overdose.[29][30]
Effects on materials
DEET is an effective solvent,[7] and may dissolve some watch crystals,[4] plastics, rayon, spandex, other synthetic fabrics, and painted or varnished surfaces including nail polish. It also may act as a plasticizer by remaining inside some formerly hard plastics, leaving them softened and more flexible.
Effects on the environment
Though DEET is not expected to bioaccumulate, it has been found to have a slight toxicity for fresh-water fish such as rainbow trout[31] and tilapia,[32] and it also has been shown to be toxic for some species of freshwater zooplankton.[33] DEET has been detected at low concentrations in water bodies as a result of production and use, such as in the Mississippi River and its tributaries, where a 1991 study detected levels varying from 5 to 201 ng/L.[34]
See also
References
- 1 2 US 2408389, Gertler, Samuel, "N,N-diethylbenzamide as an insect repellent", published 1946-10-01
- ↑ Katz, Tracy (February 14, 2008). "Insect repellents: Historical perspectives and new developments". Journal of the American Academy of Dermatology. 58 (5): 865–871. doi:10.1016/j.jaad.2007.10.005. PMID 18272250. Retrieved 2015-08-16.
- ↑ Committee on Gulf War and Health: Literature Review of Pesticides and Solvents (2003). Gulf War and Health: Volume 2. Insecticides and Solvents. Washington, D.C.: National Academies Press. ISBN 978-0-309-11389-2.
- 1 2 3 4 Kitchen; Lawrence. "The role of the United States military in the development of vector control products, including insect repellents, insecticides, and bed nets". Journal of Vector Ecology. 34 (1): 50–61. doi:10.1111/j.1948-7134.2009.00007.x.
- ↑ Wang, Benjamin J-S. (1974). "An interesting and successful organic experiment (CEC)". J. Chem. Educ. 51 (10): 631. doi:10.1021/ed051p631.2.
- ↑ Donald L. Pavia (2004). Introduction to organic laboratory techniques (Google Books excerpt). Cengage Learning. pp. 370–376. ISBN 978-0-534-40833-6.
- 1 2 3 Anna Petherick (2008-03-13). "How DEET jams insects' smell sensors". Nature News. Retrieved 2008-03-16.
- ↑ Mathias Ditzen, Maurizio Pellegrino, Leslie B. Vosshall (2008). "Insect Odorant Receptors Are Molecular Targets of the Insect Repellent DEET". Sciencexpress. 319 (5871): 1838–42. doi:10.1126/science.1153121. PMID 18339904.
- 1 2 Syed, Z.; Leal, WS (2008). "Mosquitoes smell and avoid the insect repellent DEET". Proc. Natl. Acad. Sci. USA. 105 (36): 13598–603. doi:10.1073/pnas.0805312105. PMC 2518096. PMID 18711137.
- ↑ Fox, Maggie; David Wiessler (Aug 18, 2008). "For mosquitoes, DEET just plain stinks". Washington. Reuters. Archived from the original on August 11, 2011. Retrieved August 11, 2011.
- ↑ Tsitsanou, K.E.; et al. (2012). "Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: implications for structure-based design of novel mosquito repellents". Cell Mol Life Sci. 69 (2): 283–97. doi:10.1007/s00018-011-0745-z. PMID 21671117.
- ↑ Stanczyk, Nina M.; Brookfield, John F. Y.; Field, Linda M.; Logan, James G. (2013). Vontas, John, ed. "Aedes aegypti Mosquitoes Exhibit Decreased Repellency by DEET following Previous Exposure". PLoS ONE (published 20 February 2013). 8 (2): e54438. doi:10.1371/journal.pone.0054438. Lay summary – BBC news (21 February 2013)
- ↑ Kain, Pinky; Boyle, Sean Michael; Tharadra, Sana Khalid; Guda, Tom; Pham, Christine; Dahanukar, Anupama; Ray, Anandasankar (2013). "Odour receptors and neurons for DEET and new insect repellents". Nature. 502: 507–512. doi:10.1038/nature12594. PMC 3927149.
- 1 2 Xu, Pingxi; Choo, Young-Moo; De La Rosa, Alyssa; Leal, Walter S. (2014). "Mosquito odorant receptor for DEET and methyl jasmonate". Proceedings of the National Academy of Sciences. 111 (46): 16592–16597. doi:10.1073/pnas.1417244111. ISSN 0027-8424. PMC 4246313.
- ↑ American Academy of Pediatrics, “Summer Safety Tips,” Dec 2, 2017 https://www.healthychildren.org/English/safety-prevention/at-play/Pages/Summer-Safety-Tips-Staying-Safe-Outdoors.aspx
- ↑ Record in the Household Products Database of NLM
- ↑ Matsuda, Brent M.; Surgeoner, Gordon A.; Heal, James D.; Tucker, Arthur O.; Maciarello, Michael J. (1996). "Essential oil analysis and field evaluation of the citrosa plant "Pelargonium citrosum" as a repellent against populations of Aedes mosquitoes". Journal of the American Mosquito Control Association. 12 (1): 69–74. PMID 8723261.
- ↑ David Williamson (3 July 2002). "Independent study: DEET products superior for fending off mosquito bites" (Press release). University of North Carolina.
- ↑ "Protection against Mosquitoes, Ticks, Fleas and Other Insects and Arthropods". Travelers' Health - Yellow Book. Centers for Disease Control and Prevention. 2009-02-05.
- 1 2 3 "Insect Repellent Use and Safety". West Nile Virus. Centers for Disease Control and Prevention. 2007-01-12.
- ↑ "Bug spray poisoning". U.S. National Library of Medicine. October 2015. Retrieved 2016-06-25.
- ↑ Comparative Efficacy of Insect Repellents Against MosquitoesMark S. Fradin, M.D., and Jonathan F. Day, Ph.D., New England Journal of Medicine, 2002 http://www.nejm.org/doi/pdf/10.1056/NEJMoa011699
- ↑ "Reregistration Eligibility Decision: DEET" (PDF). U.S. Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances. September 1998. pp. 39–40. Archived from the original (PDF) on October 21, 2012. Retrieved 2012-09-08.
- ↑ "DEET". Pesticide Information Profile. EXTOXNET. October 1997. Retrieved 2007-09-26.
- ↑ "Insect Repellents". Healthy Living. Health Canada. August 2009. Archived from the original on 2010-04-11. Retrieved 2010-07-09.
- ↑ "Re-evaluation Decision Document: Personal insect repellents containing DEET (N,N-diethyl-m-toluamide and related compounds)" (PDF). Consumer Product Safety. Health Canada. 2002-04-15. Retrieved 2010-07-09.
- ↑ "How to choose the best bug repellent". Best Health. Reader's Digest Association, Inc. Retrieved June 14, 2016.
‘Anything intended for topical use only shouldn’t be going into the body,’ says Xiaochen Gu, a professor at the University of Manitoba’s faculty of pharmacy, who led the study.
- ↑ Moss (1996). "Synergism of Toxicity of N,N-Diethyl-m-toluamide to German Cockroaches (Othoptera: Blattellidae) by Hydrolytic Enzyme Inhibitors". J. Econ. Entomol. 89 (5): 1151–1155. doi:10.1093/jee/89.5.1151. PMID 17450648.
- ↑ Tenenbein, M. (1987). "Severe toxic reactions and death following the ingestion of diethyltoluamide-containing insect repellents". J Amer Med Asso. 258: 1509–11. doi:10.1001/jama.258.11.1509. PMID 3625951.
- ↑ Baselt RC (2014). Disposition of toxic drugs and chemicals in man, 10th edition. Seal Beach, Ca.: Biomedical Publications. p. 650. ISBN 978-0-9626523-9-4.
- ↑ U.S. Environmental Protection Agency. 1980. Office of Pesticides and Toxic Substances. N,N-diethyl-m-toluamide (Deet) Pesticide Registration Standard. December 1980. 83 pp.
- ↑ Mathai, AT; Pillai, KS; Deshmukh, PB (1989). "Acute toxicity of deet to a freshwater fish, Tilapia mossambica : Effect on tissue glutathione levels". Journal of Environmental Biology. 10 (2): 87–91. Archived from the original on 2007-11-07.
- ↑ J. Seo; Y. G. Lee; S. D. Kim; C. J. Cha; J. H. Ahn; H. G. Hur (2005). "Biodegradation of the Insecticide N,N-Diethyl-m-Toluamide by Fungi: Identification and Toxicity of Metabolites". Archives of Environmental Contamination and Toxicology. 48 (3): 323–328. doi:10.1007/s00244-004-0029-9. PMID 15750774.
- ↑ "Errol Zeiger, Raymond Tice, Brigette Brevard, (1999) N,N-Diethyl-m-toluamide (DEET) [134-62-3] - Review of Toxicological Literature" (PDF). Archived from the original (PDF) on October 9, 2012. Retrieved July 20. Check date values in:
|accessdate=(help)
Further reading
- M. S. Fradin (1 June 1998). "Mosquitoes and Mosquito Repellents: A Clinician's Guide". Ann Intern Med. 128 (11): 931–940. doi:10.7326/0003-4819-128-11-199806010-00013. PMID 9634433.
External links
| Wikimedia Commons has media related to DEET. |
- DEET General Fact Sheet - National Pesticide Information Center
- DEET Technical Fact Sheet - National Pesticide Information Center
- West Nile Virus Resource Guide - National Pesticide Information Center
- Health Advisory: Tick and Insect Repellents, New York State
- US Centers for Disease Control information on DEET
- US Environmental Protection Agency information on DEET
- Review of scientific literature on DEET (from a RAND Corporation report on Gulf War illnesses)