Cytochrome d
Cytochrome d is a protein that belongs to the Cytochrome family (electron-transporting proteins). Concretely, it is an enzyme – a protein that catalyses a chemical reaction. Cytochrome d is also known as cytochrome/heme a2. It is found in plenty of aerobic bacteria, especially when it has grown with a limited oxygen supply. The bacteria species in which it is more commonly found are Escherichia coli and Aerobacter aerogenes.[1]
General functions
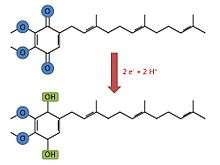
Cytochrome d is, as other proteins of its family, a membrane-bound hemeprotein, but unlike cytochromes a and b, cytochrome D has a tetrapyrrolic chelate of iron as a prosthetic group[2] instead of a heme A or heme B group. It is a dimeric protein, which means that is compound of two subunits (CydA[3] and CydB[4]). At the same time, cytochrome d is part of the cytochrome bd-I terminal oxidase which catalyse the two electron oxidation of ubiquinol. This process is an oxidative phosphorylation that oxidizes the ubiquinol-8 to ubiquinone. The chemical reaction followed by this process is:
Ubiquinol-8 + O2 = Ubiquinone-8 + H2O[5]
By a similar reaction, it also catalyses the reduction of oxygen to water, which involves 4 electrons.
Cytochrome d in protein complexes
Generally, in protein complexes, it gives an absorption band of approximately 636 nm or 638 nm, depending on the cytochrome d form. If it is oxidized, the band has a length of 636 nm, and a 638 nm length if it is reduced. It is commonly associated to certain prosthetic groups when found in multiple subunit complexes. Detecting cytochrome d as Fe(II) pyridine alkaline hemachrome is very difficult because the stability under these conditions is limited. If cytochrome d is pulled out of the protein complex and placed in ether containing from 1 to 5 % of HCl, it gives a different absorption band (603 nm, in the oxidized form).[6]
Cytochrome d in Escherichia coli
The cytochrome d complex from E. coli is a heterodimer found in the bacterial cytoplasmic bilayer, where it develops the function of oxidase of the respiratory chain.[7] Cytochrome d presents two subunits in this organism (I and II, abbreviated CydA and CydB), which were analyzed by genetic methods including alkaline phosphatase gene fusions. These fusions were done in vivo and in vitro, which implied the utilization of the transposon TnphoA in the in vivo method. The in vitro method consisted on a construction. In the end, 48 isolated fusions helped to determine the activities of the specific alkaline phosphatase in cells. The basis of the 2D models for each subunit was developed thanks to the data of these fusions and information from other resources. This basis also defines how these subunits fold in the E. coli membrane, specially in the inner one.[8]
Cytochrome d in Azotobacter vinelandii
Azotobacter vinelandii is a nitrogen-fixing bacteria which is known by its high respiratory rate among aerobic organisms. Some physiological studies postulate that cytochrome d functions as a terminal oxidase in the membranes of this organism, taking part in the electron transport system. The studies characterized the different genes in the two subunits. A very extensive homology with CydA and CydB of the E. coli was found in these studies.[9]
Cytochrome bd
Cytochrome bd is a tri-heme oxidase as it is compound by cytochromes b558, b595 and d. Its main function is the reduction of O2 to H2O. It is thought that it uses a di-heme active site, which is formed by the hemes of cytochromes b595 and d. These two cytochromes are considered high-spin complexes, what is directly related to the electrons’ spin. While other respiratory terminal oxidases which catalyze that same reaction have a heme-copper active site and use a proton pump, cytochrome bd has an active site with iron instead of copper and need no proton pump as they can produce a proton-motion force themselves.[10]
References
- ↑ Cytochrome d group
- ↑ Cytochrome+d at the US National Library of Medicine Medical Subject Headings (MeSH)
- ↑ Caulobacter crescentus CB15 Enzyme: cytochrome d ubiquinol oxidase subunit I
- ↑ Caulobacter crescentus CB15 Enzyme: cytochrome d ubiquinol oxidase subunit II
- ↑ Cytochrome d ubiquinol oxidase subunit 1
- ↑ Cytochrome d group
- ↑ Michael J. Miller, Robert B. Gennis. The Cytochrome d Complex Is a Coupling Site in the Aerobic Respiratory Chain of Escherichia coli. The Journal of Biological Chemistry Vol.260 No.26 (1985)
- ↑ Escherichia coli K-12 substr. MG1655 Transporter: cytochrome bd-I terminal oxidase
- ↑ Jones CW, Redfearn ER. The cytochrome system of Azotobacter vinelandii. Biochim Biophys Acta. 1967 Sep 6;143(2):340–353
- ↑ Vitaliy B. Borisov, Michael I. Verkhovsky. Accommodation of CO in the di-heme active site of cytochrome bd terminal oxidase from Escherichia coli. Journal of Inorganic Biochemistry 118 (2013) 65–67