Chemical trap
In chemistry, a chemical trap is a chemical compound that is used to detect a unstable chemical compounds.[1] The method relies on efficiency of bimolecular reactions with reagents to produce a more easily characterize trapped product. In some cases, the trapping agent is used in large excess.
Case studies
Cyclobutadiene
A famous example is the detection of cyclobutadiene released upon oxidation of cyclobutadieneiron tricarbonyl. When this degradation is conducted in the presence of an alkyne, the cyclobutadiene is trapped as a bicyclohexadiene. The requirement for this trapping experiment is that the oxidant (ceric ammonium nitrate) and the trapping agent be mutually compatible.
 Trapping of cyclobutadiene as its stable Diels-Alder adduct.[2]
Trapping of cyclobutadiene as its stable Diels-Alder adduct.[2]
Benzyne
Chemical traps have often been applied to the interception of benzyne.[3][4] The thermolysis of diphenylzirconacene requires the presence of a donor ligand (e.g., trimethylphosphine to facilitate the isolation of a benzyne complex:
- Cp2ZrPh2 + PMe3 → Cp2Zr(C6H4)(PMe3) + PhH
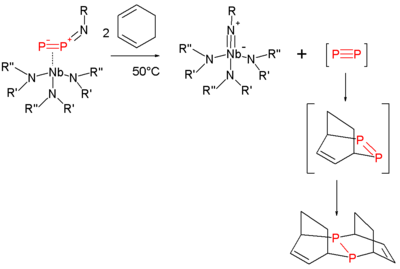
Diphosphorus
Diphosphorus is an old target of chemists since it is the heavy analogue of N2. Its fleeting existence is inferred by the controlled degradation of certain niobium complexes in the presence of trapping agents. Again, a Diels-Alder strategy is employed in the trapping:[5]
Related meanings
In some cases, chemical trap is used to detect or infer a compound when present at concentrations below its detection limit or is present in a mixture, where other components interfere with its detection. The trapping agent, for example a dye, reacts with the chemical to be detected, giving a product that is more easily detected.
References
- ↑ March, Jerry (1985), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (3rd ed.), New York: Wiley, ISBN 0-471-85472-7
- ↑ G. F. Emerson, L. Watts, R. Pettit (1965). "Cyclobutadiene- and Benzocyclobutadiene-Iron Tricarbonyl Complexes". J. Am. Chem. Soc. 87: 131–133. doi:10.1021/ja01079a032.
- ↑ Wentrup, Curt; Blanch, Rodney; Briehl, Horst; Gross, Gerhard (1988). "Benzyne, cyclohexyne, and 3-azacyclohexyne and the Problem of Cycloalkyne versus Cycloalkylideneketene Genesis". Journal of the American Chemical Society. 110: 1874–80. doi:10.1021/ja00214a034.
- ↑ Louis F. Fieser, Makhluf J. Haddadin (1966). "1,2,3,4-Tetraphenylnaphthalene". Org. Synth. 46: 107. doi:10.15227/orgsyn.046.0107.
- ↑ Piro, Nicholas A.; Figueroa, Joshua S.; McKellar, Jessica T.; Cumnins, Christopher C. (1 September 2006). "Triple-Bond Reactivity of Diphosphorus Molecules". Science. 313 (5791): 1276–1279. Bibcode:2006Sci...313.1276P. doi:10.1126/science.1129630. PMID 16946068. Retrieved 21 July 2013.