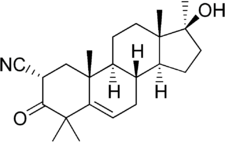
Cyanoketone
 | |
| Clinical data | |
|---|---|
| Synonyms | Cyanotrimethylandrostenolone; CTM; 2α-Cyano-4,4',17α-trimethylandrost-5-en-17β-ol-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C23H33NO2 |
| Molar mass | 355.51 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
Cyanoketone, also known as 2α-cyano-4,4',17α-trimethylandrost-5-en-17β-ol-3-one (CTM),[1] is a synthetic androstane steroid and a steroidogenesis inhibitor which is used in scientific research.[2][3][4] On account of its structural similarity to pregnenolone, cyanoketone binds to and acts as a potent, selective, and irreversible inhibitor of 3β-hydroxysteroid dehydrogenase (3β-HSD),[1][5] an enzyme that is responsible for the conversion of pregnenolone into progesterone, 17α-hydroxypregnenolone into 17α-hydroxyprogesterone, DHEA into androstenedione, and androstenediol into testosterone.[2][3][6] As such, cyanoketone inhibits the production of both gonadal and adrenal steroids, including progesterone,[5] androgens, estrogens, and corticosteroids.[3][6] The drug is too toxic for therapeutic use in humans, and so has been used instead exclusively as a research tool.[2][3]
See also
References
- 1 2 I. Chester Jones; I. W. Henderson (22 October 2013). General, Comparative and Clinical Endocrinology of the Adrenal Cortex. Elsevier Science. pp. 136–. ISBN 978-1-4832-5980-2.
- 1 2 3 John A. Thomas; Edward J. Keenan (6 December 2012). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 280–. ISBN 978-1-4684-5036-1.
- 1 2 3 4 John A. Thomas; Edward J. Keenan (6 December 2012). Principles of Endocrine Pharmacology. Springer Science & Business Media. pp. 11–12. ISBN 978-1-4684-5036-1.
- ↑ Di Prisco, C. L.; Materazzi, G.; Scattolini, B. (1971). "Inhibition of steroidogenesis by cyanoketone". Gynecologic and Obstetric Investigation. 2 (1): 309–315. doi:10.1159/000301873. PMID 4266510.
- 1 2 Barry D. Bavister (6 December 2012). Preimplantation Embryo Development. Springer Science & Business Media. pp. 30–. ISBN 978-1-4613-9317-7.
- 1 2 David O. Norris; James A. Carr (4 May 2013). Vertebrate Endocrinology. Academic Press. pp. 71–. ISBN 978-0-12-396465-6.