Capped octahedral molecular geometry
| Capped octahedral molecular geometry | |
|---|---|
 | |
| Examples | MoF7− |
| Point group | C3v |
| Coordination number | 7 |
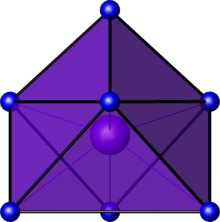
In chemistry, the capped octahedral molecular geometry describes the shape of compounds where seven atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a gyroelongated triangular pyramid. This shape has C3v symmetry and is one of the three common shapes for heptacoordinate transition metal complexes, along with the pentagonal bipyramid and the capped trigonal prism.[1]
Examples of the capped octahedral molecular geometry are the heptafluoromolybdate (MoF7−) and the heptafluorotungstate (WF7−) ions.[2][3]
References
- ↑ Roald. Hoffmann; Barbara F. Beier; Earl L. Muetterties; Angelo R. Rossi (1977). "Seven-coordination. A molecular orbital exploration of structure, stereochemistry, and reaction dynamics". Inorganic Chemistry. 16 (3): 511–522. doi:10.1021/ic50169a002.
- ↑ Kaupp, Martin (2001). ""Non-VSEPR" Structures and Bonding in d(0) Systems". Angew Chem Int Ed Engl. 40 (1): 3534–3565. doi:10.1002/1521-3773(20011001)40:19<3534::AID-ANIE3534>3.0.CO;2-#.
- ↑ Zhenyang Lin; Ian Bytheway (1996). "Stereochemistry of Seven-Coordinate Main Group and d0 Transition Metal Molecules". Inorganic Chemistry. 35 (3): 594–603. doi:10.1021/ic950271o.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.