Calcium imaging
Calcium imaging is a scientific technique usually carried out in research which is designed to show the calcium (Ca2+) status of an isolated cell, tissue or medium. Calcium imaging techniques take advantage of so-called calcium indicators, fluorescent molecules that can respond to the binding of Ca2+ ions by changing their fluorescence properties. Two main classes of calcium indicators exist: chemical indicators and genetically encoded calcium indicators (GECI). Calcium imaging can be used to optically probe intracellular calcium in living animals.[1] This technique has allowed studies of calcium signalling in a wide variety of cell types and neuronal activity in hundreds of neurons and glial cells within neuronal circuits .
Chemical indicators
Chemical indicators are small molecules that can chelate calcium ions. All these molecules are based on an EGTA homologue called BAPTA, with high selectivity for calcium (Ca2+) ions versus magnesium (Mg2+) ions.
This group of indicators includes fura-2, indo-1, fluo-3, fluo-4, Calcium Green-1.
These dyes are often used with the chelator carboxyl groups masked as acetoxymethyl esters, in order to render the molecule lipophilic and to allow easy entrance into the cell. Once this form of the indicator is in the cell, cellular esterases will free the carboxyl groups and the indicator will be able to bind calcium. The free acid form of the dyes (i.e. without the acetoxymethyl ester modification) can also be directly injected into cells via a microelectrode or micropipette which removes uncertainties as to the cellular compartment holding the dye (the acetoxymethyl ester can also enter the endoplasmic reticulum and mitochondria). Binding of a Ca2+ ion to a fluorescent indicator molecule leads to either an increase in quantum yield of fluorescence or emission/excitation wavelength shift. Individual chemical Ca2+ fluorescent indicators are utilized for cytosolic calcium measurements in a wide variety of cellular preparations. The first real time (video rate) Ca2+ imaging was carried out in 1986 in cardiac cells using intensified video cameras.[2] Later development of the technique using laser scanning confocal microscopes revealed sub-cellular Ca2+ signals in the form of Ca2+ sparks and Ca2+ blips. Relative responses from a combination of chemical Ca2+ fluorescent indicators were also used to quantify calcium transients in intracellular organelles such as mitochondria.[3]
Calcium imaging, also referred to as calcium mapping, is also used to perform research on myocardial tissue.[4] Calcium mapping is a ubiquitous technique used on whole, isolated hearts such as mouse, rat, and rabbit species.
Genetically encoded calcium indicator
These indicators are fluorescent proteins derived from green fluorescent protein (GFP) or its variants (e.g. circularly permuted GFP, YFP, CFP), fused with calmodulin (CaM) and the M13 domain of the myosin light chain kinase, which is able to bind CaM. Alternatively, variants of GFP are fused with the calcium binding protein troponin C (TnC), applying the mechanism of FRET (Förster Resonance Energy Transfer) for signal modulation.
Genetically encoded indicators do not need to be loaded onto cells, instead the genes encoding for these proteins can be easily transfected to cell lines. It is also possible to create transgenic animals expressing the dye in all cells or selectively in certain cellular subtypes. GECIs have been used in the studies of neuron[5],[6] T-cell,[7] cardiomyocyte,[8] etc.
| GECI | Year | Sensing | Reporting | Precursor |
|---|---|---|---|---|
| Cameleons[9] | 1997 | Calmodulin | FRET pair: BFP or CFP, and GFP or YFP | - |
| FIP-CBSM[10] | 1997 | Calmodulin | FRET pair: BFP and RFP | - |
| Pericams[11] | 2000 | Calmodulin | cpGFP | - |
| GCaMP[12] | 2000 | Calmodulin | cpEGFP | - |
| TN-L15[13] | 2004 | Modified chicken skeletal muscle troponin C | FRET pair: YFP (Citrine) and CFP (Cerulean) | - |
| TN-humTnC[13] | 2004 | Human cardiac troponin C | FRET pair: YFP (Citrine) and CFP (Cerulean) | - |
| TN-XL[14] | 2006 | Modified chicken skeletal muscle troponin C | FRET pair: permuted YFP (Citrine) and CFP (Cerulean) | TN-L15 |
| TN-XXL[15] | 2008 | Modified csTnC in TN-XL | FRET pair: permuted YFP (Citrine) and CFP (Cerulean) | TN-XL |
| Twitch's[16] | 2014 | Troponin C | FRET pair (various of two FPs) | - |
Usage
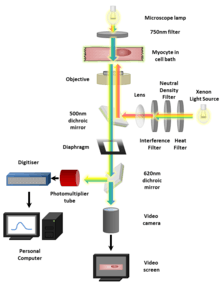
Regardless of the type of indicator used the imaging procedure is generally very similar. Isolated cells loaded with an indicator or expressing it in the case of GECI, can be viewed using a fluorescence microscope and captured by a Scientific CMOS (sCMOS)[17] camera or CCD camera. Confocal and two-photon microscopes provide improved sectioning ability so that calcium signals can be resolved in micro domains or (for example) synaptic boutons. Images are analyzed by measuring fluorescence intensity changes for a single wavelength or two wavelengths expressed as a ratio (ratiometric indicators). The derived fluorescence intensities and ratios are plotted against calibrated values for known Ca2+ levels to learn Ca2+ concentration. Light field microscopy methods[18] extend functional readout of neural activity capabilities in 3D volumes.
References
- ↑ Stosiek, Christoph; Garaschuk, Olga; Holthoff, Knut; Konnerth, Arthur (2003). "In vivo two-photon calcium imaging of neuronal networks". Proceedings of the National Academy of Sciences. 100 (12): 7319–24. Bibcode:2003PNAS..100.7319S. doi:10.1073/pnas.1232232100. JSTOR 3139475. PMC 165873. PMID 12777621.
- ↑ Cannell, M. B.; Berlin, J. R.; Lederer, W. J. (1987-01-01). "Intracellular calcium in cardiac myocytes: calcium transients measured using fluorescence imaging". Society of General Physiologists Series. 42: 201–214. ISSN 0094-7733. PMID 3505361.
- ↑ Ivannikov, Maxim V.; Macleod, Gregory T. (2013). "Mitochondrial Free Ca2+ Levels and Their Effects on Energy Metabolism in Drosophila Motor Nerve Terminals". Biophysical Journal. 104 (11): 2353–61. doi:10.1016/j.bpj.2013.03.064. PMC 3672877. PMID 23746507.
- ↑ Jaimes, Rafael; Walton, Richard D.; Pasdois, Phillipe Lionel Claude; Bernus, Olivier; Efimov, Igor R.; Kay, Matthew W (2016). "A Technical Review of Optical Mapping of Intracellular Calcium within Myocardial Tissue". American Journal of Physiology. Heart and Circulatory Physiology. 310: ajpheart.00665.2015. doi:10.1152/ajpheart.00665.2015. PMID 27016580.
- ↑ Afshar Saber, Wardiya; Gasparoli, Federico M.; Dirks, Marjet G.; Gunn-Moore, Frank J.; Antkowiak, Maciej (2018). "All-Optical Assay to Study Biological Neural Networks". Frontiers in Neuroscience. 12. doi:10.3389/fnins.2018.00451. ISSN 1662-453X.
- ↑ Kovalchuk, Yury; Homma, Ryota; Liang, Yajie; Maslyukov, Anatoliy; Hermes, Marina; Thestrup, Thomas; Griesbeck, Oliver; Ninkovic, Jovica; Cohen, Lawrence B. (2015-02-19). "In vivo odourant response properties of migrating adult-born neurons in the mouse olfactory bulb". Nature Communications. 6: 6349. doi:10.1038/ncomms7349. ISSN 2041-1723. PMID 25695931.
- ↑ Mues, Marsilius; Bartholomäus, Ingo; Thestrup, Thomas; Griesbeck, Oliver; Wekerle, Hartmut; Kawakami, Naoto; Krishnamoorthy, Gurumoorthy (2013-06-01). "Real-time in vivo analysis of T cell activation in the central nervous system using a genetically encoded calcium indicator". Nature Medicine. 19 (6): 778–783. doi:10.1038/nm.3180. ISSN 1546-170X. PMID 23685843.
- ↑ Shinnawi, Rami; Huber, I; Maizels, L; Shaheen, N; Gepstein, A; Arbel, G; Tijsen, A; Gepstein, L (2015). "Monitoring human-induced pluripotent stem cell-derived cardiomyocytes with genetically encoded calcium and voltage fluorescent reporters". Stem Cell Reports. 5 (4): 582–596. doi:10.1016/j.stemcr.2015.08.009. PMC 4624957. PMID 26372632.
- ↑ Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J. M.; Adams, J. A.; Ikura, M.; Tsien, R. Y. (1997-08-28). "Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin". Nature. 388 (6645): 882–887. doi:10.1038/42264. ISSN 0028-0836. PMID 9278050.
- ↑ Romoser, V. A.; Hinkle, P. M.; Persechini, A. (1997-05-16). "Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators". The Journal of Biological Chemistry. 272 (20): 13270–13274. doi:10.1074/jbc.272.20.13270. ISSN 0021-9258. PMID 9148946.
- ↑ Nagai, T.; Sawano, A.; Park, E. S.; Miyawaki, A. (2001-03-13). "Circularly permuted green fluorescent proteins engineered to sense Ca2+". Proceedings of the National Academy of Sciences of the United States of America. 98 (6): 3197–3202. doi:10.1073/pnas.051636098. ISSN 0027-8424. PMC 30630. PMID 11248055.
- ↑ Nakai, J.; Ohkura, M.; Imoto, K. (2001-02-01). "A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein". Nature Biotechnology. 19 (2): 137–141. doi:10.1038/84397. ISSN 1087-0156. PMID 11175727.
- 1 2 Heim, Nicola; Griesbeck, Oliver (2004-04-02). "Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein". The Journal of Biological Chemistry. 279 (14): 14280–14286. doi:10.1074/jbc.M312751200. ISSN 0021-9258. PMID 14742421.
- ↑ Mank, Marco; Reiff, Dierk F.; Heim, Nicola; Friedrich, Michael W.; Borst, Alexander; Griesbeck, Oliver (2006-03-01). "A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change". Biophysical Journal. 90 (5): 1790–1796. doi:10.1529/biophysj.105.073536. ISSN 0006-3495. PMC 1367327. PMID 16339891.
- ↑ Mank, Marco; Santos, Alexandre Ferrão; Direnberger, Stephan; Mrsic-Flogel, Thomas D.; Hofer, Sonja B.; Stein, Valentin; Hendel, Thomas; Reiff, Dierk F.; Levelt, Christiaan (2008-09-01). "A genetically encoded calcium indicator for chronic in vivo two-photon imaging". Nature Methods. 5 (9): 805–811. doi:10.1038/nmeth.1243. ISSN 1548-7091. PMID 19160515.
- ↑ Thestrup, Thomas; Litzlbauer, Julia; Bartholomäus, Ingo; Mues, Marsilius; Russo, Luigi; Dana, Hod; Kovalchuk, Yuri; Liang, Yajie; Kalamakis, Georgios (2014-02-01). "Optimized ratiometric calcium sensors for functional in vivo imaging of neurons and T lymphocytes". Nature Methods. 11 (2): 175–182. doi:10.1038/nmeth.2773. ISSN 1548-7105. PMID 24390440.
- ↑ Jeffrey P. Nguyen, Frederick B. Shipley, Ashley N. Linder, George S. Plummer, Mochi Liu, Sagar U. Setru, Joshua W. Shaevitz, and Andrew M. Leifer (2016-02-23). "Whole-brain calcium imaging with cellular resolution in freely behaving Caenorhabditis elegans". PNAS 113 (8) E1074-E1081. https://doi.org/10.1073/pnas.1507110112
- ↑ Pégard, N.; Liu, H.; Antipa, N; Gerlock, M.; Adesnik, H.; Waller, L. (2016). "Compressive light-field microscopy for 3D neural activity recording". Optica. 3: 517–524. doi:10.1364/optica.3.000517.
Further reading
- Nuccitelli, Richard, ed. (1994). "A Practical guide to the study of calcium in living cells". Methods in Cell Biology. Boston: Academic Press. ISBN 978-0-12-564141-8.