Burette

A burette (also buret) is a graduated glass tube with a tap at one end, for delivering known volumes of a liquid, especially in titrations. It is a long, graduated glass tube, with a stopcock at its lower end and a tapered capillary tube at the stopcock's outlet. The flow of liquid from the tube to the burette tip is controlled by the stopcock valve. There are two main types of burette; the volumetric burette and the Piston burette or Digital burette.
A volumetric burette delivers measured volumes of liquid. Piston burettes are similar to syringes, but with a precision bore and a plunger. Piston burettes may be manually operated or may be motorized.[1] A weight burette delivers measured weights of a liquid.[2]
Overview
A burette is a volumetric measuring glassware which is used in analytical chemistry for accurate dispensing of variable, and for measuring the volume of a liquid, especially of one of the reagents in a titration.[3] In a titration of an acid and base solution, the burette can be filled with acid. By turning the tap in a perpendicular direction, the tap can be opened, which then allows the acid in the tube to be able to be added in a stream or drop by drop into a flask. The experiment is taken out by gradually filling a liquid sample(s). Titrant or titrated solution is a good example. Burette support the process by its long narrow tubed with a stopcock at the taper end. The addition will halt when there the correspondence point is set up.[4]
Burettes can perform titrations precisely for delivering a volumetric measurement of a fluid.[5] The burette tube carries graduated marks from which the dispensed volume of the liquid can be determined.[6] Compared to pipette, greater precision can be attained by using a burette.[7]
The burette is used to measure a volume of a substance in a specific amount, but different from measuring cylinder as its graduations measure from top to bottom. Therefore, the difference between the starting and the final volume is equal to the amount dispensed.[8] The precision and control of the burette over other means of adding solution is beneficial for using in titration in order to add titrant to the titrated solution.[9]
Volumetric Burette
Volumetric burette can be glass or plastic straight tube with a graduation scale. At the tip of burette, there are a stopcock and valve to control the flow of the chemical solution. The barrel of the stopcock can be made of glass or the plastic PTFE. Stopcocks with glass barrels need to be lubricated with Vaseline or a specialized grease. Burettes are manufactured for specific tolerances, designated as class A or B and this also is etched on the glass.
 Black stripped technique
Black stripped technique
Burette reading
The amount of solution added in or drained out needed to be read correctly by observing at eye level straight to the bottom of "Meniscus" for most solutions. Before reading the data, the bubbles must all been removed from the Burette otherwise the data will be inaccurately measured.[10] Also, reading the data at the eye level means looking straight at the bottom of the meniscus. The initial and final volumes collected will be calculated for the difference in volume which equal to the total volume of solution drained out of the Burette. The difference in volume can be calculated by taking the difference of final volume and initial volume[11] Using the Burette with a colorless solution is sometimes difficult to observe the bottom of the meniscus so Black Strip Technique[12] can help to accurately observe and measure the number on the scale. Moreover, the number should be reported in two decimal places, which can be done more easily by using the Black strip Technique. The black strip can be written with pen on the normal white paper or it can be printed it out. However, it is necessary to use white color paper as the background, in order to make the scale readable.[12]
Specification
Specification or product specification is used as an identification of volumetric burette[13] for example nominal volume, volume unit, error limit, accuracy class of the burette and manufacture's related details. Specification is directly association with the usage of each laboratory equipment including burette. Therefore, it is necessary to be able to understand each of specification in details in order to perform the accurate experiment. Nominal volume, error and units are the basic knowledge in order to distinguish the amount of solution delivered from the burette in unit of ml or cm3. Another specification for burette is called calibration marked as TD or Ex stand for "Calibration to Deliver". It indicates that this burette is better used to delivery purpose which the amount will be correspond to the volume as specified[14] The accuracy classes of equipment also shown in the specification of burette as well and it includes class A and class B. Class A is more preferred than Class B when volumetric accuracy is important for the accuracy of the experiment with accuracy up to 0.1 percent compared to 0.2 percent in Class B burette.[15]

Digital Burette
Digital burettes are based on a syringe design. The barrel and plunger may be made of glass. With liquids that corrode glass, including solutions of alkali, the barrel and plunger may be made of polyethylene or another resistant plastic material. The barrel is held in a fixed position and the plunger is moved incrementally either by turning a ratcheted wheel by hand, or by means of a step motor. The volume is shown on a digital display. A high-precision syringe may be used to deliver very precise aliquots. Motorized Digital Burettes may be controlled by a computer; for example, a titration may be recorded digitally and then subject to numerical processing to find the titer at an end-point.
History
The first burette was invented in 1845 by the French chemist Étienne Ossian Henry (1798–1873).[16][17] In 1855, the German chemist Karl Friedrich Mohr (1806–1879) presented an improved version of Henry's burette, having graduations inscribed on the tube of the burette.[18]
The word "burette" was coined in 1824 by the French chemist Joseph Louis Gay-Lussac (1778–1850).[19]
Additional images
- Meniscus
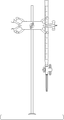

 Burette with Ring stand
Burette with Ring stand plastic stopcock used in glass volumetric burette
plastic stopcock used in glass volumetric burette
References
- ↑ Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel's Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, ISBN 0-582-22628-7 Section 3.12, p.79, "Burettes"
- ↑ Redman, H. N. (1963). "An improved type of weight burette for use in volumetric analysis". Analyst. 88: 654–655. Bibcode:1963Ana....88..654R. doi:10.1039/AN9638800654.
- ↑ Chauhan, Yogesh. "Burettes, Laboratory Glassware". EzineArticles. Missing or empty
|url=(help);|access-date=requires|url=(help) - ↑ "Titration". britannica. 2017.
- ↑ "Burettes". chem.yorku.ca.
- ↑ "Acids and Alkalis". gcsescience.
- ↑ Skoog, D.A.; West, D.M.; Holler, F.J. (2000). Analytical Chemistry: An Introduction, seventh edition. Emily Barrosse. pp. 44–45. ISBN 0-03-020293-0.
- ↑ "Burette - Preproom.org". Retrieved 2017-05-23.
- ↑ "Laboratory volumetric glassware used in titration - burette, pipette, ASTM E287-02 standard specification". www.titrations.info. Retrieved 2017-05-23.
- ↑ Henrickson, Charles H.; Byrd, Larry C.; Hunter, Norman W. (2000). "1". A laboratory for General, Organic & Biochemistry. The Mcgraw-Hill Companies, Inc.
- ↑ Sienko, Michell J.; Plane, Robert A.; Marcus, Stanley T. (1984). Experimental Chemistry. McGraw-Hill. Inc. p. 16.
- 1 2 Seely, Oliver. "Helpful Hints on the Use of a Burette". www.csudh.edu. Retrieved 2017-06-09.
- ↑ "LabWare LIMS v6 Help". limshelp.labware.com. Retrieved 2017-06-20.
- ↑ "Measuring Volume". www.harpercollege.edu. Retrieved 2017-06-20.
- ↑ "Burette – Jaytec Glass". www.jaytecglass.co.uk. Retrieved 2017-06-20.
- ↑ Henry, O. (1845). "Nouvelles expériences sur l'essai des potasses du commerce et appareil dit potassimètre pour l'effectuer" [New experiments on the assay of commercial potash and an apparatus called a "potassimeter" to perform it]. Journale de Pharmacie et de Chimie. 3rd series (in French). 7: 214–222. A sketch of Henry's burette appears on p. 218. From p. 218: "AC est un tube de verre d'une longuere de 60 centimètres environ, et d'un diamètre de 4 millimètres à peu près. En A se trouve un entonnoir de verre soudé ou adapté à volunté; et en B un petite robinet en cuivre terminé par un tube capillaire. Ce robinet s'adjuste au tube par un bon bouchon et avec de la cire à cacheter. Le tube AB est fixé par deux crochets au long d'une échelle inscrite sur une planche, et cette échelle est divisé en 100 parties égales. Le tout est supporté par un pied qui permet de placer le tube AB au-dessus du vase M contenant le sel de potasse à essayer." (AC is a glass tube [that's] about 60 cm. long, and nearly 4 mm. in diameter. At A, a glass funnel is joined or fitted as desired; and at B [there is] a small copper valve ending with a capillary tube. This valve is fitted to the tube by a good cap and with sealing wax. The tube AB is fixed by two brackets along a scale [that's] inscribed on a plate, and this scale is divided into 100 equal parts. The whole is supported by a base that permits placing the tube AB above a vase M containing the potassium salt to be assayed.)
- ↑ See:
- Szabadváry, Ferenc (1986). "The history of chemical laboratory equipment". Periodica Polytechnica Chemical Engineering. 30 (1–2): 77–95. See p. 87.
- Szabadváry, Ferenc (1966). History of Analytical Chemistry. Translated by Gyula Svehla. Oxford, England: Permagon Press. p. 237.
- Christophe, R. (1971). "L'analyse volumétrique de 1790 à 1860. Caractéristiques et importance industrielle. Evolution des instruments" [Volumetric analysis from 1790–1860. Characteristics and industrial importance. Evolution of instruments.]. Revue d'histoire des sciences (in French). 24 (1): 25–44. From p. 38: " … il préfigure bien ses descendants actuelles … " ( … it [i.e., Henry's burette] foreshadows well its modern descendants … )
- ↑ Mohr, Karl Friedrich (1855). Lehrbuch der chemisch-analytischen Titrirmethode … , part 1 [Textbook of analytical chemistry titration methods …] (in German). Braunschweig, (Germany): Friederich Vieweg und Sohn. pp. 2–20. Page 3 shows Mohr's burette; page 12 shows a burette with a glass stopcock (Glasshahn).
- ↑ Gay-Lussac, Joseph Louis (1824). "Instruction sur l'essai du chlorure de chaux" [Instructions on the assaying of chlorinated lime]. Annales de chimie et de physique. 2nd series (in French). 26: 162–175. On p. 171, Gay-Lussac describes various figures that appear in a plate (illustration) that accompanies the article. From p. 171: " I, burette destinée à mesurer la teinture d'épreuve: … " (I, burette intended to measure the test dye: … )
External links
- Using a Burette from ChemLab at Dartmouth College demonstrating how to use a burette correctly
- Use of the Buret