Branaplam
 | |
| Clinical data | |
|---|---|
| Synonyms | LMI070; NVS-SM1 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
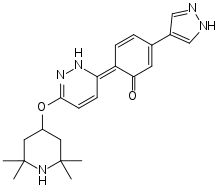
| Formula | C22H27N5O2 |
| Molar mass | 393.491 g/mol |
| 3D model (JSmol) | |
| |
| |
Branaplam, also known as LMI070 and NVS-SM1, is a highly potent, selective and orally active small molecule experimental drug being developed by Novartis to treat spinal muscular atrophy (SMA). It is a pyridazine derivative that works by increasing the amount of functional survival of motor neuron protein produced by the SMN2 gene through modifying its splicing pattern.[1][2]
As of March 2017, branaplam is in a phase-II clinical trial in children with SMA type 1.[3][4]
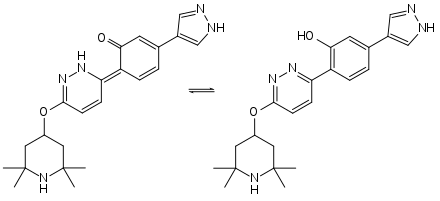
Keto-enol tautomerism of branaplam
References
- ↑ Palacino, James; Swalley, Susanne E; Song, Cheng; Cheung, Atwood K; Shu, Lei; Zhang, Xiaolu; Van Hoosear, Mailin; Shin, Youngah; Chin, Donovan N; Keller, Caroline Gubser; Beibel, Martin; Renaud, Nicole A; Smith, Thomas M; Salcius, Michael; Shi, Xiaoying; Hild, Marc; Servais, Rebecca; Jain, Monish; Deng, Lin; Bullock, Caroline; McLellan, Michael; Schuierer, Sven; Murphy, Leo; Blommers, Marcel J J; Blaustein, Cecile; Berenshteyn, Frada; Lacoste, Arnaud; Thomas, Jason R; Roma, Guglielmo; et al. (2015). "SMN2 splice modulators enhance U1–pre-mRNA association and rescue SMA mice". Nature Chemical Biology. 11 (7): 511. doi:10.1038/nchembio.1837. PMID 26030728.
- ↑ "LMI070". SMA News Today. Retrieved 2017-03-10.
- ↑ "An Open Label Study of LMI070 in Type 1 Spinal Muscular Atrophy (SMA)". ClinicalTrials.gov. Retrieved 2017-03-10.
- ↑ "Novartis Releases Update on LMI070 (Branaplam) Clinical Trial". CureSMA. 2017-09-20. Retrieved 2017-10-07.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.