Borophene
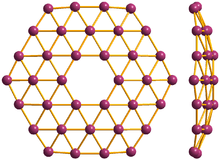
36° borophene, front and side view
Borophene is a proposed crystalline allotrope of boron. One unit consists of 36 atoms arranged in a 2-dimensional sheet with a hexagonal hole in the middle.[1][2] Another form made in 2015 is a buckled two dimensional sheet on silver.[3]
Theory
Computational studies suggested that extended borophene sheets with partially filled hexagonal holes are stable.[4][5] Global minimum searches for B−
36 lead to a quasiplanar structure with a central hexagonal hole. Borophene is predicted to be fully metallic.[1]
Borophene is analogous to graphene in that it is expected to form extended sheets. The latter is a semi-metal, implying that borophene may be a better conductor.[6] The boron-boron bond is also nearly as strong as graphene’s carbon-carbon bond.[1] At the atomic-cluster scale, pure boron forms simple planar molecules and cage-like fullerenes.[7]
Boron is adjacent to carbon in the periodic table and has similar valence orbitals. Unlike carbon, boron cannot form a honeycomb hexagonal framework (like graphene) because of its electron deficiency.[1]
History
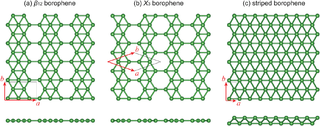
In 2014 a research team at Brown University, led by Lai-Sheng Wang, showed that the structure of B
36 was not only possible but highly stable.[2][6][8] Photoelectron spectroscopy revealed a relatively simple spectrum, suggesting a symmetric cluster. Neutral B
36 is the smallest boron cluster to have sixfold symmetry and a perfect hexagonal vacancy, and it can be viewed as a potential basis for extended two-dimensional boron sheets.[1]
In 2015 a research team synthesized borophene on silver surfaces under ultrahigh-vacuum conditions. Atomic-scale characterization, supported by theoretical calculations, revealed structures reminiscent of fused boron clusters with multiple scales of anisotropic, out-of-plane buckling. Unlike bulk boron allotropes, borophene shows metallic characteristics that are consistent with predictions of a highly anisotropic, 2D metal.[7] Notably, the atomic structure of borophene on Ag(111) was revealed to be the same as the one predicted by an earlier theory on the same substrate.[9]
See also
| Look up borophene in Wiktionary, the free dictionary. |
References
- 1 2 3 4 5 "Will 'borophene' replace graphene as a better conductor of electrons?". KurzweilAI. February 5, 2014. Retrieved February 5, 2014.
- 1 2 Piazza, Z. A.; Hu, H. S.; Li, W. L.; Zhao, Y. F.; Li, J.; Wang, L. S. (2014). "Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets". Nature Communications. 5: 3113. Bibcode:2014NatCo...5E3113P. doi:10.1038/ncomms4113. PMID 24445427.
- ↑ Mannix, A. J.; Zhou, X.-F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X.; Fisher, B. L.; Santiago, U.; Guest, J. R.; et al. (17 December 2015). "Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs". Science. 350 (6267): 1513–1516. Bibcode:2015Sci...350.1513M. doi:10.1126/science.aad1080. PMC 4922135. PMID 26680195.
- ↑ Tang, Hui & Ismail-Beigi, Sohrab (2007). "Novel Precursors for Boron Nanotubes: The Competition of Two-Center and Three-Center Bonding in Boron Sheets". Physical Review Letters. 99 (11): 115501. arXiv:0710.0593. Bibcode:2007PhRvL..99k5501T. doi:10.1103/PhysRevLett.99.115501. PMID 17930448.
- ↑ Gonzalez Szwacki, N. (2008). "Boron Fullerenes: A First-Principles Study". Nanoscale Research Letters. 3 (2): 49–54. arXiv:0711.0767. Bibcode:2008NRL.....3...49G. doi:10.1007/s11671-007-9113-1.
- 1 2 "New boron nanomaterial may be possible". Brown University. January 27, 2014. Retrieved March 9, 2013.
- 1 2 Mannix, A. J.; Zhou, X.-F.; Kiraly, B.; Wood, J. D.; Alducin, D.; Myers, B. D.; Liu, X.; Fisher, B. L.; Santiago, U. (2015-12-18). "Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs". Science. 350 (6267): 1513–1516. Bibcode:2015Sci...350.1513M. doi:10.1126/science.aad1080. PMC 4922135. PMID 26680195.
- ↑ Johnson, Dexter (January 28, 2014). "'Borophene' Might Be Joining Graphene in the 2-D Material Club". IEEE Spectrum. Retrieved March 9, 2013.
- ↑ Zhang, Zhuhua; Yang, Yang; Gao, Guoying; Yakobson, Boris I. (2015). "Two‐Dimensional Boron Monolayers Mediated by Metal Substrates". Angewandte Chemie International Edition. 127 (44): 13022. doi:10.1002/ange.201505425.