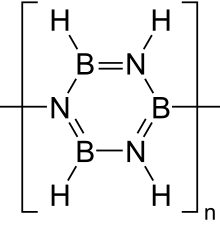
Borazine
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,3,5,2,4,6-Triazatriborinane (only preselected[1]) | |||
| Other names
Borazine Cyclotriborazaneborazol Inorganic benzene Borazole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.169.303 | ||
PubChem CID |
|||
| |||
| |||
| Properties | |||
| B3H6N3 | |||
| Molar mass | 80.50 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.81 g/cm3 | ||
| Melting point | −58 °C (−72 °F; 215 K) | ||
| Boiling point | 53 °C (127 °F; 326 K) (55 °C at 105 Pa) | ||
| -49.6·10−6 cm3/mol | |||
| Hazards | |||
| NFPA 704 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Borazine is an inorganic compound with the chemical formula (BH)3(NH)3. In this cyclic compound, the three BH units and three NH units alternate. The compound is isoelectronic and isostructural with benzene. Like benzene, borazine is a colourless liquid.[2] For this reason borazine is sometimes referred to as "inorganic benzene".
Synthesis
The compound was reported in 1926 by the chemists Alfred Stock and Erich Pohland by a reaction of diborane with ammonia.[3]
Borazine is synthesized from diborane and ammonia in a 1:2 ratio at 250–300 °C with a conversion of 50%.
- 3 B2H6 + 6 NH3 → 2 B3H6N3 + 12 H2
An alternative more efficient route begins with lithium borohydride and ammonium chloride:
- 3 LiBH4 + 3 NH4Cl → B3H6N3 + 3 LiCl + 9 H2
In a two-step process to borazine, boron trichloride is first converted to trichloroborazine:
- 3 BCl3 + 3 NH4Cl → Cl3B3H3N3 + 9 HCl
The B-Cl bonds are subsequently converted to B-H bonds:
- 2 Cl3B3H3N3 + 6 NaBH4 → 2 B3H6N3 + 3 B2H6 + 6 NaCl
Properties
Borazine is a colourless liquid with an aromatic smell. In water it hydrolyzes to boric acid, ammonia, and hydrogen. Borazine, with a standard enthalpy change of formation ΔHf of −531 kJ/mol, is thermally very stable.
Structure
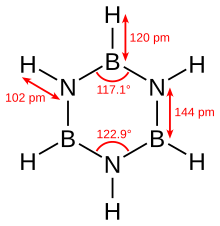
Borazine is isoelectronic with benzene and has similar connectivity, so it is sometimes referred to as "inorganic benzene". This comparison is not rigorously valid due to the electronegativity difference between boron and nitrogen. X-ray crystallographic structural determinations show that the bond lengths within the borazine ring are all equivalent at 1.429 Å, a property shared by benzene.[4] However, the borazine ring does not form a perfect hexagon. The bond angle is 117.1° at the boron atoms and 122.9° at the nitrogens, giving the molecule distinct symmetry.
The electronegativity of boron (2.04 on the Pauling scale) compared to that of nitrogen (3.04) and also the electron deficiency on the boron atom and the lone pair on nitrogen favor alternative mesomer structures for borazine.
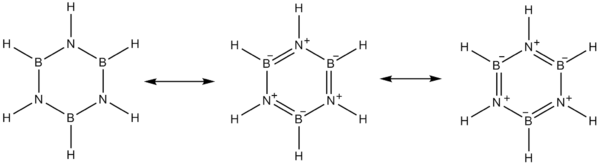
Boron behaves as a Lewis acid and nitrogen behaves as a Lewis base.
Aromaticity
Due to its similarities to benzene, there have been a number of computational and experimental analyses of borazine's aromaticity. The number of pi electrons in borazine obeys the 4n + 2 rule, and the B-N bond lengths are equal, which suggests the compound may be aromatic. The electronegativity difference between boron and nitrogen, however, creates an unequal sharing of charge which results in bonds with greater ionic character, and thus it is expected to have poorer delocalization of electrons than the all-carbon analog.

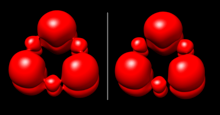
Natural Bond Orbitals (NBO)
Natural Bond Orbital (NBO) analysis suggests weak aromaticity in borazine.[5] In the NBO model, B-N bonds in the ring are slightly displaced from the nuclear axes, and B and N have large differences in charge. Natural chemical shielding (NCS) analysis provides some further evidence for aromaticity based on a contribution of the B-N π bond to magnetic shielding. Computations based on NBO orbitals show that this π bond allows for weak ring current which somewhat counteracts a magnetic field simulated at the center of the borazine ring. A small ring current does suggest some delocalization.
Atoms in Molecules (AIM)
Atoms in Molecules (AIM) analysis can examine degree of covalency in bonding through examination of critical points in the negative Laplacian of electron density for a given molecule. In the case of borazine, bond critical points are found to lie extremely close to the boron atoms, indicating electrons are concentrated around the nitrogens, and thus there is a large difference in charge between B and N. Furthermore the Laplacians of these bond critical points are positive, implying that these bonds are largely ionic. AIM analysis thus suggests poor covalency and thus poor electron sharing, indicating only weak aromaticity, if any.
Electron Localization Function (ELF)
Topological analysis of bonding in borazine by the Electron Localization Function (ELF) indicates that borazine can be described as a π aromatic compound. However, the bonding in borazine is less delocalized than in benzene based on a difference in bifurcation values of the electron basins. Larger bifurcation values indicate better electron delocalization, and it is argued that when this bifurcation value is greater than 0.70, the delocalization is sufficient to designate a compound aromatic.[6] For benzene, this value is 0.91, but the borazine π system bifurcates at the ELF value 0.682.[7] This is caused by the difference in electronegativity between B and N, which produces a weaker bond interaction than the C-C interaction in benzene, leading to increased localization of electrons on the B-H and N-H units. The bifurcation value is slightly below the limit of 0.70 which suggests moderate aromaticity.
Reactivity
Although often compared with benzene, borazine is far more reactive. With hydrogen chloride it forms an adduct, whereas benzene is unreactive toward HCl.
- B3N3H6 + 3 HCl → B3N3H9Cl3
- Addition reaction of borazine with hydrogen chloride
- B3N3H9Cl3 + NaBH4 → (BH4N)3
- Reduction with sodium borohydride
The addition reaction with bromine does not require a catalyst. Borazines undergo nucleophilic attack at boron and electrophilic attack at nitrogen. Heating borazine at 70 °C expels hydrogen with formation of a borazinyl polymer or polyborazylene, in which the monomer units are coupled in a para fashion by new boron-nitrogen bonds. Boron nitride can be prepared by heating polyborazylene to 1000 °C. Borazines are also starting materials for other potential ceramics such as boron carbonitrides. Borazine can also be used as a precursor to grow boron nitride thin films on surfaces, such as the nanomesh structure which is formed on rhodium.
Polyborazylene has been proposed as a recycled hydrogen storage medium for hydrogen fuel cell vehicle applications, using a "single pot" process for digestion and reduction to recreate ammonia borane.[8]
Among other B-N type compounds mixed amino-nitro substituted borazines have been predicted to outperform carbon based explosives such as CL-20.[9][10]
Related compounds
Carborazine is another six-membered aromatic ring with two carbon atoms, two nitrogen atoms and two boron atoms in opposing pairs.[11][12]
See also
- 1,2-Dihydro-1,2-azaborine — an aromatic chemical compound with properties intermediate between benzene and borazine.
- Iminoborane
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 968. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Duward Shriver; Peter Atkins (2010). Inorganic Chemistry (Fifth ed.). New York: W. H. Freeman and Company. p. 328. ISBN 978-1429218207.
- ↑ Stock, Alfred; Pohland, Erich (October 1926). "Borwasserstoffe, VIII. Zur Kenntnis des B2H6 und des B5H11" [Boric acid solution, VIII Regarding knowledge of B2H6 and B5H11]. Berichte (in German). 59 (9): 2210–2215. doi:10.1002/cber.19260590906.
- ↑ Boese, R.; Maulitz, A. H.; Stellberg, P. (1994). "Solid-State Borazine: Does it Deserve to be Entitled "Inorganic Benzene"?". Chemische Berichte. 127 (10): 1887–1889. doi:10.1002/cber.19941271011.
- ↑ Shen, W.; Li, M.; Li, Y.; Wang, S. (2007). "Theoretical study of borazine and its derivatives". Inorganica Chim. Acta. 360: 619–624. doi:10.1016/j.ica.2006.08.028.
- ↑ Santos, J. C.; Tiznado, W.; Fuentealba, P. (2004). "Sigma–pi separation of the electron localization function and aromaticity". The Journal of Chemical Physics. 120 (4): 1670–1673. doi:10.1063/1.1635799.
- ↑ Islas, R.; Chamorro, E.; Robles, J.; Heine, T.; Santos, J. C.; Merino, G. (2007). "Borazine: to be or not to be aromatic". Struct. Chem. 18: 833–839. doi:10.1007/s11224-007-9229-z.
- ↑ Davis, B. L.; Dixon, D. A.; Garner, E. B.; Gordon, J. C.; Matus, M. H.; Scott, B.; Stephens, F. H. (2009). "Efficient Regeneration of Partially Spent Ammonia Borane Fuel". Angewandte Chemie International Edition. 48 (37): 6812–6816. doi:10.1002/anie.200900680. PMID 19514023.
- ↑ Koch, E.-C; Klapötke, T. M. (2012). "Boron-Based High Explosives". Propellants, Explosives, Pyrotechnics. 37: 335–344. doi:10.1002/prep.201100157.
- ↑ Kervyn, Simon; Fenwick, Oliver; Di Stasio, Francesco; Shin, Yong Sig; Wouters, Johan; Accorsi, Gianluca; Osella, Silvio; Beljonne, David; Cacialli, Franco; Bonifazi, Davide (10 June 2013). "Polymorphism, Fluorescence, and Optoelectronic Properties of a Borazine Derivative". Chemistry: A European Journal. 19 (24): 7771–7779. doi:10.1002/chem.201204598.
- ↑ Srivastava, Ambrish Kumar; Misra, Neeraj (2015). "Introducing "carborazine" as a novel heterocyclic aromatic species". New Journal of Chemistry. 39 (4): 2483–2488. doi:10.1039/c4nj02089h.
- ↑ Bonifazi, Davide; Fasano, Francesco; Lorenzo-Garcia, M. Mercedes; Marinelli, Davide; Oubaha, Hamid; Tasseroul, Jonathan (2015). "Boron–nitrogen doped carbon scaffolding: organic chemistry, self-assembly and materials applications of borazine and its derivatives". Chem. Commun. 51 (83): 15222–15236. doi:10.1039/C5CC06611E.
- Larry G. Sneddon; Mario G. L. Mirabelli; Anne T. Lynch; Paul J. Fazen; Kai Su; Jeffrey S. Beckdon (1991). "Polymeric precursors to boron based ceramics" (PDF). Pure Appl. Chem. 63 (3): 407–410. doi:10.1351/pac199163030407.
- Jong-Kyu Jeon; Yuko Uchimaru; Dong-Pyo Kim (2004). "Synthesis of Novel Amorphous Boron Carbonitride Ceramics from the Borazine Derivative Copolymer via Hydroboration". Inorg. Chem. 43 (16): 4796–4798. doi:10.1021/ic035254a.
- P. Paetzold (1991). "New perspectives in boron-nitrogen chemistry - I" (PDF). Pure Appl. Chem. 63 (3): 345–350. doi:10.1351/pac199163030345.
- Islas, Rafael (2007). "Borazine: to be or not to be aromatic". Structural Chemistry. 18 (6): 833–839. doi:10.1007/s11224-007-9229-z.
External links
![]()