Bis(trimethylsilyl)amine
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,1,1-Trimethyl-N-(trimethylsilyl)silanamine | |||
| Other names
Bis(trimethylsilyl)azane Bis(trimethylsilyl)amine 1,1,1,3,3,3-Hexamethyldisilazane (no longer recommended[1]) Hexamethyldisilazane (ambiguous) | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | HMDS | ||
| 635752 | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.012.425 | ||
| EC Number | 213-668-5 | ||
| MeSH | Hexamethylsilazane | ||
PubChem CID |
|||
| RTECS number | JM9230000 | ||
| UN number | 2924, 3286 | ||
| |||
| |||
| Properties | |||
| C6H19NSi2 | |||
| Molar mass | 161.40 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 0.77 g cm−3 | ||
| Melting point | −78 °C (−108 °F; 195 K) | ||
| Boiling point | 126 °C (259 °F; 399 K) | ||
| slow hydrolysis | |||
Refractive index (nD) |
1.4080 | ||
| Hazards | |||
| Safety data sheet | External MSDS | ||
| NFPA 704 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Bis(trimethylsilyl)amine (also known as hexamethyldisilazane, or HMDS) is an organosilicon compound with the molecular formula [(CH3)3Si]2NH. The molecule is a derivative of ammonia with trimethylsilyl groups in place of two hydrogen atoms. This colorless liquid is a reagent and a precursor to bases that are popular in organic synthesis and organometallic chemistry.
Preparation and reactions
Bis(trimethylsilyl)amine is prepared by treatment of trimethylsilyl chloride with ammonia:[2]
- 2 (CH3)3SiCl + 3 NH3 → [(CH3)3Si]2NH + 2 NH4Cl
The product is usually handled using air-free techniques since it hydrolyzes slowly in humid air.
Alkali metal bis(trimethylsilyl)amides result from the deprotonation of bis(trimethylsilyl)amine. For example, lithium bis(trimethylsilyl)amide (LiHMDS) is prepared using n-butyllithium:
- [(CH3)3Si]2NH + BuLi → [(CH3)3Si]2NLi + C4H10
Together with sodium bis(trimethylsilyl)amide (NaHMDS) and potassium bis(trimethylsilyl)amide (KHMDS), LiHMDS is used as a non-nucleophilic base.
Reactions
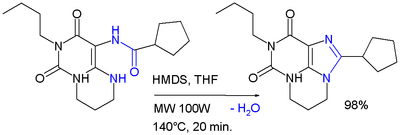
One of the uses of HMDS is as a reagent in condensation reactions of heterocyclic compounds such as in the microwave synthesis of a derivative of xanthine:[3]
HMDS can be used to convert alcohols into trimethylsilyl ethers. HMDS can be used to silylate laboratory glassware and make it hydrophobic, or automobile glass, just as Rain-X does.
In gas chromatography, HMDS can be used to silylate OH groups of organic compounds to increase volatility, this way enabling GC-analysis of chemicals that are otherwise non-volatile.
Other uses
In photolithography, HMDS is often used as an adhesion promoter for photoresist. Best results are obtained by applying HMDS from the gas phase on heated substrates.[4]
In electron microscopy, HMDS can be used as an alternative to critical point drying during sample preparation.[5]
In pyrolysis-gas chromatography-mass spectrometry, HMDS is added to the analyte to create silylated diagnostic products during pyrolysis, in order to enhance detectability of compounds with polar functional groups.[6]
See also
References
- ↑ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 135. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ↑ Robert C. Osthoff; Simon W. Kantor (1957). "Organosilazane Compounds". Inorg. Synth. Inorganic Syntheses. 5: 55–64. doi:10.1002/9780470132364.ch16. ISBN 978-0-470-13236-4.
- ↑ Burbiel JC, Hockemeyer J, Müller CE (2006). "Microwave-assisted ring closure reactions: Synthesis of 8-substituted xanthine derivatives and related pyrimido- and diazepinopurinediones". Beilstein J Org Chem. 2: 20. doi:10.1186/1860-5397-2-20. PMC 1698928. PMID 17067400.
- ↑ Cornell NanoScale Science & Technology Facility. "CNF - Photolithography Resist Processes and Capabilities". Retrieved 2008-01-29.
- ↑ Bray DF, Bagu J, Koegler P (1993). "Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens". Microsc. Res. Tech. 26 (6): 489–95. doi:10.1002/jemt.1070260603. PMID 8305726.
- ↑ Giuseppe Chiavari; Daniele Fabbri & Silvia Prati (2001). "Gas chromatographic–mass spectrometric analysis of products arising from pyrolysis of amino acids in the presence of hexamethyldisilazane". Journal of Chromatography A. 922 (1–2): 235–241. doi:10.1016/S0021-9673(01)00936-0. PMID 11486868.