Biohydrogen

Biohydrogen is H2 that is produced biologically.[1] Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass.[2] Many challenges characterize this technology, including those intrinsic to H2, such as storage and transportation of a noncondensible gas. Hydrogen producing organisms are poisoned by O2. Yields of H2 are often low.
Biochemical principles
The main reactions involve fermentation of sugars. Important reactions start with glucose, which is converted to acetic acid:[3]
- C6H12O6 + 2 H2O → 2 CH3CO2H + 2 CO2 + 4 H2
A related reaction gives formate instead of carbon dioxide:
- C6H12O6 + 2 H2O → 2 CH3CO2H + 2 HCO2H + 2 H2
These reactions are exergonic by 216 and 209 kcal/mol, respectively.
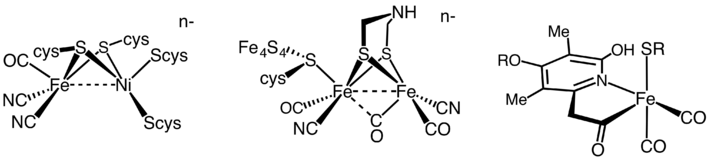
H2 production is catalyzed by two hydrogenases. One is called [FeFe]-hydrogenase; the other is called [NiFe]-hydrogenase. Many organisms express these enzymes. Notable examples are members of the genera Clostridium, Desulfovibrio, Ralstonia, and the pathogen Helicobacter. E. coli is the workhorse for genetic engineering of hydrogenases.[4]
It has been estimated that 99% of all organisms utilize dihydrogen (H2). Most of these species are microbes and their ability to use H2 as a metabolite arises from the expression of H2 metalloenzymes known as hydrogenases.[5] Hydrogenases are sub-classified into three different types based on the active site metal content: iron-iron hydrogenase, nickel-iron hydrogenase, and iron hydrogenase.
History
In 1933, Marjory Stephenson and her student Stickland reported that cell suspensions catalysed the reduction of methylene blue with H2. Six years later, Hans Gaffron observed that the green photosynthetic alga Chlamydomonas reinhardtii, would sometimes produce hydrogen. Gaffron and many other scientists failed to elucidate how it induced hydrogen production. In the late 1990s Anastasios Melis discovered that deprivation of sulfur induces the alga to switch from the production of oxygen (normal photosynthesis) to the production of hydrogen. He found that the enzyme responsible for this reaction is hydrogenase, but that the hydrogenase lost this function in the presence of oxygen. Melis also discovered that depleting the amount of sulfur available to the algae interrupted their internal oxygen flow, allowing the hydrogenase an environment in which it can react, causing the algae to produce hydrogen.[6] Chlamydomonas moewusii is also a promising strain for the production of hydrogen.[7]
Industrial hydrogen
Competing for biohydrogen, at least for commercial applications, are many mature industrial processes. Hydrogen is usually derived from fossil fuels by steam reforming of natural gas - sometimes referred to as steam methane reforming (SMR) - is the most common method of producing bulk hydrogen at about 95% of the world production.[8][9][10]
References
- ↑ M. Rögner, ed. (2015). Biohydrogen. De Gruyter. ISBN 978-3-11-033673-3.
- ↑ Y.-H. Percival Zhang "Hydrogen Production from Carbohydrates: A Mini-Review" in "Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass" ACS Symposium Series, 2011, Volume 1067, pages=203-216.
- ↑ Thauer, R. K., "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson", Microbiology 1998, 144, 2377-2406. doi:10.1099/00221287-144-9-2377
- ↑ Cammack, R.; Frey, M.; Robson, R. (2001). Hydrogen as a Fuel: Learning from Nature. London: Taylor & Francis.
- ↑ Lubitz, Wolfgang; Ogata, Hideaki; Rüdiger, Olaf; Reijerse, Edward (2014). "Hydrogenases". Chemical Reviews. 114: 4081-4148. doi:10.1021/cr4005814.
- ↑ Reengineering Algae To Fuel The Hydrogen Economy
- ↑ Melis A & Happe T (2001). "Hydrogen Production. Green Algae as a Source of Energy". Plant Physiol. 127 (3): 740–748. doi:10.1104/pp.010498. PMC 1540156. PMID 11706159.
- ↑ P. Häussinger, R. Lohmüller, A. M. Watson, "Hydrogen, 2. Production" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.o13_o03
- ↑ Ogden, J.M. (1999). "Prospects for building a hydrogen energy infrastructure". Annual Review of Energy and the Environment. 24: 227–279. doi:10.1146/annurev.energy.24.1.227.
- ↑ "Hydrogen Production: Natural Gas Reforming". Department of Energy. Retrieved 6 April 2017.
