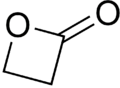
''beta''-Propiolactone
 | |
 | |
| Names | |
|---|---|
| IUPAC names
Oxetan-2-one 3-Hydroxypropanoic acid lactone | |
| Other names
Propiolactone β-Propiolactone 2-Oxetanone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.309 |
| EC Number | 200-340-1 |
| KEGG | |
| UNII | |
| |
| |
| Properties | |
| C3H4O2 | |
| Molar mass | 72.06 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | slightly sweet[1] |
| Density | 1.1460 g/cm3 |
| Melting point | −33.4 °C (−28.1 °F; 239.8 K) |
| Boiling point | 162 °C (324 °F; 435 K) (decomposes) |
| 37 g/100 mL | |
| Solubility in organic solvents | Miscible |
| Vapor pressure | 3 mmHg (25°C)[1] |
Refractive index (nD) |
1.4131 |
| Hazards | |
| Flash point | 74 °C; 165 °F; 347 K [1] |
| Explosive limits | 2.9%-?[1] |
| US health exposure limits (NIOSH): | |
PEL (Permissible) |
OSHA-Regulated carcinogen[1] |
REL (Recommended) |
Ca[1] |
IDLH (Immediate danger) |
Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
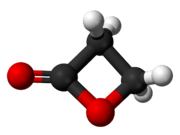
β-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and miscible with ethanol, acetone, diethyl ether and chloroform.[2][3] The word propiolactone usually refers to this compound, although it may also refer to α-propiolactone.
Preparation
β-Propiolactone is prepared industrially by the reaction of formaldehyde and ketene:[4]
- CH2O + CH2=C=O → (CH2CH2CO2)
In the research laboratory, propiolactones have been produced by the carbonylation of epoxides.[5]
Reactions and applications
β-Propiolactone readily polymerizes even at room temperature.
It reacts with many nucleophiles in a ring-opening reactions. With water hydrolyze occurs to produce 3-hydroxypropionic acid (hydracryclic acid). Ammonia gives the β-alanine, which is a commercial process.[4]
Propiolactone was once widely produced as an intermediate in the production of acrylic acid and its esters. That application has been largely displaced in favor of safer and less expensive alternatives. β-Propiolactone is an excellent sterilizing and sporicidal agent, but its carcinogenicity precludes that use.[2] The principal use of propiolactone is an intermediate in the synthesis of other chemical compounds.[4]
Safety
β-Propiolactone is "reasonably anticipated to be a human carcinogen" (IARC, 1999).[2] It is one of 13 "OSHA-regulated carcinogens," chemicals regarded occupational carcinogens by the Occupational Safety and Health Administration, despite not having an established permissible exposure limit.[6]
Biodegradation
Acidovorax sp., Variovorax paradoxus, Sphingomonas paucimobilis, Rhizopus delemar and thermophilic Streptomyces sp. can degrade β-propiolactone.
See also
- 3-Oxetanone, an isomer of β-propiolactone
- Malonic anhydride (2,4-oxetanone)
- α-Propiolactone
References
- 1 2 3 4 5 6 7 "NIOSH Pocket Guide to Chemical Hazards #0528". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "β-Propiolactone CAS No. 57-57-8" - US Department of Health and Human Services, Report on Carcinogens, National Toxicology Program, Thirteenth Edition, 2 October 2014. Accessed on 2015-01-03.
- ↑ Merck Index, 12th Edition, entry 8005.
- 1 2 3 Karlheinz Miltenberger "Hydroxycarboxylic Acids, Aliphatic" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a13_507
- ↑ John W. Kramer, Daniel S. Treitler, Geoffrey W. Coates (2009). "Low Pressure Carbonylation of Epoxides to β-Lactones". Org. Synth. 86: 287. doi:10.15227/orgsyn.086.0287.
- ↑ "Appendix B - Thirteen OSHA-Regulated Carcinogens" - Centers for Disease Control and Prevention. Accessed on 2013-11-06.