Benzoin condensation
The benzoin condensation is a reaction (often called a condensation reaction, for historical reasons) between two aldehydes, particularly benzaldehyde. The reaction generally occurs between aromatic aldehydes or glyoxals. The reaction is formally a 1,2-addition reaction and is catalyzed by nucleophiles such as a cyanide anion or an N-heterocyclic carbene (usually thiazolium salts). The reaction product is an aromatic acyloin, with benzoin as the parent compound.[1] An early version of the reaction was developed in 1832 by Justus von Liebig and Friedrich Woehler during their research on bitter almond oil.[2] The catalytic version of the reaction involving cyanide was developed by Nikolay Zinin in the late 1830s,[3][4] and the reaction mechanism for this organic reaction was proposed in 1903 by A. J. Lapworth.[5]
Reaction mechanism
In the first step in this reaction, the cyanide anion (as sodium cyanide) reacts with the aldehyde in a nucleophilic addition. Rearrangement of the intermediate results in polarity reversal of the carbonyl group, which then adds to the second carbonyl group in a second nucleophilic addition. Proton transfer and elimination of the cyanide ion affords benzoin as the product. This is a reversible reaction, which means that the distribution of products is determined by the relative thermodynamic stability of the products and starting material.
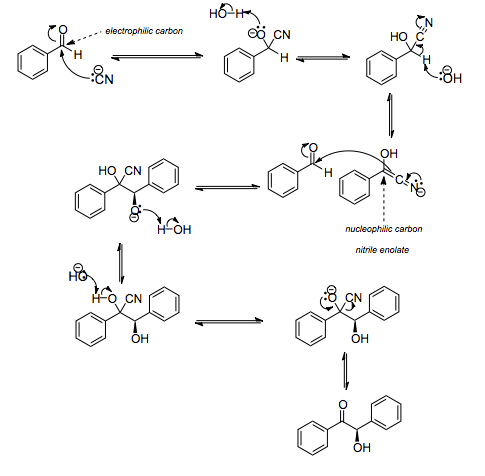
The cyanide ion serves three different purposes in the course of this reaction. It acts as a nucleophile, facilitates proton abstraction, and is also the leaving group in the final step. The benzoin condensation is in effect a dimerization and not a condensation because a small molecule like water is not released in this reaction. For this reason the reaction is also called a benzoin addition. In this reaction, the two aldehydes serve different purposes; one aldehyde donates a proton and one aldehyde accepts a proton. Some aldehydes can only donate protons, such as 4-Dimethylaminobenzaldehyde whereas benzaldehyde is both a proton acceptor and donor. In this way it is possible to synthesise mixed benzoins, i.e. products with different groups on each half of the product. However, care should be taken to match a proton donating aldehyde with a proton accepting aldehyde to avoid undesired homo-dimerization.
Scope
The reaction can be extended to aliphatic aldehydes with base catalysis in the presence of thiazolium salts; the reaction mechanism is essentially the same. These compounds are important in the synthesis of heterocyclic compounds. The analogous 1,4-addition of an aldehyde to an enone is called the Stetter reaction.
In biochemistry, the coenzyme thiamine is responsible for biosynthesis of acyloin-like compounds utilizing the benzoin condensation. This coenzyme also contains a thiazolium moiety, which on deprotonation becomes a nucleophilic carbene.
The asymmetric version of this reaction has been performed by utilizing chiral thiazolium and triazolium salts. Triazolium salts were found to give greater enantiomeric excess than thiazolium salts.[6] An example is shown below.[7]

Since the products of the reaction are thermodynamically controlled, the Retro-Benzoin Condensation can be synthetically useful. If a benzoin or acyloin can be synthesized by another method, then they can be converted into the component ketones using cyanide or thiazolium catalysts. The reaction mechanism is the same as above, but it occurs in the reverse direction. This can allow the access of ketones otherwise difficult to produce.
See also
References
- ↑ Roger Adams and C. S. Marvel (1941). "Benzoin". Organic Syntheses. ; Collective Volume, 1, p. 94
- ↑ Wöhler, Liebig; Liebig (1832). "Untersuchungen über das Radikal der Benzoesäure". Annalen der Pharmacie. 3 (3): 249–282. doi:10.1002/jlac.18320030302.
- ↑ N. Zinin (1839). "Beiträge zur Kenntniss einiger Verbindungen aus der Benzoylreihe". Annalen der Pharmacie. 31 (3): 329–332. doi:10.1002/jlac.18390310312.
- ↑ N. Zinin (1840). "Ueber einige Zersetzungsprodukte des Bittermandelöls". Annalen der Pharmacie. 34 (2): 186–192. doi:10.1002/jlac.18400340205.
- ↑ Lapworth, A. (1904). "CXXII.—Reactions involving the addition of hydrogen cyanide to carbon compounds. Part II. Cyanohydrins regarded as complex acids". Journal of the Chemical Society, Transactions. 85: 1206–1214. doi:10.1039/CT9048501206.
- ↑ Knight, Roland; Leeper, F. (1998). "Comparison of chiral thiazolium and triazolium salts as asymmetric catalysts for the benzoin condensation". J. Chem. Soc., Perkin Trans. 1: 1891–1894. doi:10.1039/A803635G.
- ↑ D. Enders, O. Niemeier & T. Balensiefer (2006). "Asymmetric Intramolecular Crossed-Benzoin Reactions by N-Heterocyclic Carbene Catalysis". Angewandte Chemie International Edition. 45 (9): 1463–1467. doi:10.1002/anie.200503885. PMID 16389609.