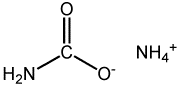
Ammonium carbamate
 | |
| Names | |
|---|---|
| Other names
ammonium aminoformate, carbamic acid ammoniate, carbamic acid ammonium salt, carbamic acid monoammonium salt[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.012.896 |
| EC Number | 214-185-2 |
| 14637 (G) | |
PubChem CID |
|
| RTECS number | EY8575000 |
| UN number | 9083 |
| |
| |
| Properties | |
| CH6N2O2 | |
| Molar mass | 78.07 g·mol−1 |
| Appearance | Colorless, rhombic crystals |
| Density | 1.38 g/cm3 (20 °C) |
| Melting point | 60 °C (140 °F; 333 K) decomposes |
| Freely soluble in water | |
| Solubility | Soluble in alcohol |
| log P | −0.47 in octanol/water |
| Vapor pressure | 492 mmHg(51 °C) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH |
151.8 kj/mol |
| Hazards | |
| Main hazards | Harmful if ingested, harmful to aquatic life, harmful if inhaled, respiatory tract irritation, skin irritation, eye irritation |
| Safety data sheet | External MSDS |
| GHS pictograms |  |
| GHS signal word | WARNING |
| NFPA 704 | |
| Flash point | 105.6 °C (222.1 °F; 378.8 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1,470 mg/kg in a rat |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ammonium carbamate is the inorganic compound with the formula NH4[H2NCO2]. This salt formed by the reaction of ammonia with carbon dioxide. This compound is a white solid that is extremely soluble in water, less so in alcohol. It is unusual in degrading at room temperature.
Preparation and structure
It is prepared by the direct reaction between liquid ammonia and dry ice (solid carbon dioxide):[2]
- 2 NH3 + CO2 → H2NCOONH4
The structure of solid ammonium carbamate has been confirmed by X-ray crystallography. The oxygen centers form hydrogen bonds to the ammonium cation.[3]
Reactions
Ammonium carbamate reverts to carbon dioxide and ammonia even as a solid:[4]
- NH2CO2NH4 → 2NH3 + CO2
It hydrates reversibly:[4]
- NH2CO2NH4 + H2O → (NH4)2CO3
Ammonium carbamate serves a key role in the formation of carbamoyl phosphate, which is necessary for both the urea cycle and the production of pyrimidines. In this enzyme-catalyzed reaction, ATP and ammonium carbamate are converted to ADP and carbamoyl phosphate:[5][6]
- ATP + NH2CO2NH4 → ADP + H2NC(O)OPO32−
Uses
The ability of ammonium carbamate to make urea was first discovered in 1870 when Bassarov heated ammonium carbamate in sealed glass tubes at temperatures ranging from 130 to 140 °C. This heating yields urea and water in an equimolar ratio.[4] A typical industrial plant that makes urea can produce up to 1500 tons a day. Ammonia and carbon dioxide is excessively fed to a synthesis reactor in this process. Ammonium carbamate is produced as an intermediate in this reactor and can then be dehydrated to urea according to the following equation:[7]
- NH2CO2NH4 → NH2CONH2 + H2O
Ammonium carbamate has also been approved by the Environmental Protection Agency as an inert ingredient present in aluminum phosphide pesticide formulations. This pesticide is commonly used for insect and rodent control in areas where agricultural products are stored. The reason for ammonium carbamate as an ingredient is to make the phosphine less flammable by freeing ammonia and carbon dioxide to dilute hydrogen phosphide formed by a hydrolysis reaction.[8]
Ammonium carbamate can also be used as a good ammoniating agent, though not nearly as strong as ammonia itself.[9]
References
- ↑ "Ammonium Carbamate" Retrieved October 12, 2012.
- ↑ Brooks, L. A.; Audrieta, L. F.; Bluestone, H.; Jofinsox, W. C. (2007). "Ammonium Carbamate". Inorg. Synth. 2: 85. doi:10.1002/9780470132333.ch23.
- ↑ J. M. Adams; R. W. H. Small (1973). "The crystal structure of ammonium carbamate". Acta Crystallogr. B29: 2317–2319. doi:10.1107/S056774087300662X.
- 1 2 3 Clark, K. G.; Gaddy, V. L.; Rist, C. E. (1933). "Equilibria in the Ammonium Carbamate-Urea-Water System". Ind. Eng. Chem. 25 (10): 1092–1096. doi:10.1021/ie50286a008.
- ↑ Goldberg, R. N. Apparent Equilibrium Constants for Enzyme-catalyzed reactions (2009). CRC Handbook of Chemistry and Physics, 7–19. Retrieved from https://www.nist.gov/manuscript-publication-search.cfm?pub_id=900943
- ↑ Phosphorus Compounds: Advances in Research and Application: 2011 Edition
- ↑ Barzagli, F., Mani, F., & Peruzzini, M. (2011). From greenhouse gas to feedstock: formation of ammonium carbamate from CO2 and NH3 in organic solvents and its catalytic conversion into urea under mild conditions. Green Chemistry, 13(5), 1267–1274
- ↑ United States Environmental Protection Agency. (2006). Inert Reassessment-Ammonium Carbamate [Data File]. Retrieved from http://www.epa.gov/opprd001/inerts/carbamate.pdf
- ↑ "Merck Index Fourteenth Addition" Retrieved October 19, 2012.
