Acrylate polymer
Acrylate polymers belong to a group of polymers which could be referred to generally as plastics. They are noted for their transparency, resistance to breakage, and elasticity. They are also commonly known as acrylics or polyacrylates. Acrylate polymer is commonly used in cosmetics such as nail polish as an adhesive.[1]
Monomers
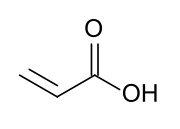
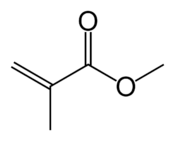
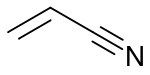
Acrylate monomers used to form acrylate polymers are based on the structure of acrylic acid, which consists of a vinyl group and a carboxylic acid ester terminus or a nitrile.[2][3] Other typical acrylate monomers are derivatives of acrylic acid, such as methyl methacrylate in which one vinyl hydrogen and the carboxylic acid hydrogen are both replaced by methyl groups, and acrylonitrile in which the carboxylic acid group is replaced by the related nitrile group.
Other examples of acrylate monomers are:
- Methacrylates[4]
- Methyl acrylate
- Ethyl acrylate
- 2-Chloroethyl vinyl ether
- 2-Ethylhexyl acrylate
- Hydroxyethyl methacrylate
- Butyl acrylate
- Butyl methacrylate
- TMPTA
Acrylic elastomers
Acrylic elastomer is a general term for a type of synthetic rubber whose main component is acrylic acid alkylester (ethyl or butyl ester).[5] Acrylic elastomer has characteristics of heat and oil resistance.
It is divided into old type and new type: Old types include ACM (copolymer of acrylic acid ester and 2-chloroethyl vinyl ether) containing chlorine and ANM (copolymer of acrylic acid ester and acrylonitrile) without chloride. Other than the slightly better water resistance of ANM, there are no physical differences; even processability is poor for both types. Since prices are also high, demand is not so high vis-à-vis the characteristics. On the other hand, the new type of acrylic rubber does not contain any chlorine despite its unclear chemical composition. Processability has been improved; most of the tackiness to rolls, as well as staining problems related to mold have been solved.
Major characteristics of acrylic rubber include heat resistance and oil resistance; it can endure a temperature of 170–180 ℃ under dry heat or in oil. Since it does not have a double bond, acrylic rubber also boasts of good weatherability and ozone resistance.
Its cold resistance is not that good, however. The saturation point is −15 ℃ for the old type and −28...−30 ℃ for the new type. In terms of vulcanization, the standard method for the old type is amine vulcanization. To minimize permanent deformation, the old type requires curing for 24 hours at a temperature of 150 ℃. On the other hand, for the new type, the press curing time and follow-up vulcanization time are significantly reduced by combining metal soap and sulfur. It has no special characteristics. The rebound resilience and abrasion resistance of the new type are poor, and even its electrical characteristics are considerably poor compared with acrylonitrile-butadiene rubber and butyl rubber.
The materials are used mainly for oil seals and packagings related to automobiles.
Other acrylic polymers
- Polymethyl methacrylate, an acrylate polymer familiar to consumers is the clear break resistant glass or sheeting sold in hardware stores as acrylic glass or under the trade name Plexiglas, among others
- Polyacrylate emulsion, water-born coating, are used as binder for outdoor and indoor "latex" house paints
- Acrylic paints as artist paints
- Acrylic fibre
- Sodium polyacrylate water-soluble thickeners, a polymer for the production of the Superabsorbent polymer (SAP) used in disposable diapers due to its high absorbency per unit mass
- Acrylic resin as pressure-sensitive adhesive
- "Super glue" is a formulation of cyanoacrylate.[6]
- PVAc copolymer emulsion adhesive of vinyl acetate (VAM) and acrylic acid (VAA)
- Polyacrylamide copolymer used as flocculation agent in water treatment
See also
References
- ↑ Erich Penzel (2000). Polyacrylates. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_157.
- ↑ Takashi Ohara, Takahisa Sato, Noboru Shimizu, Günter Prescher, Helmut Schwind, Otto Weiberg, Klaus Marten, Helmut Greim (2002). "Acrylic Acid and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH,. doi:10.1002/14356007.a01_161.pub2.
- ↑ http://pslc.ws/macrog/acrylate.htm
- ↑ Manfred Stickler, Thoma Rhein (2000). "Polymethacrylates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a21_473.
- ↑ "Archived copy". Archived from the original on 2011-06-12. Retrieved 2010-05-26.
- ↑ http://www.rsc.org/chemistryworld/2013/06/cyanoacrylate-superglue-adhesive-glue-podcast