Salen ligand
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
2,2′-Ethylenebis(nitrilomethylidene)diphenol, N,N′-Ethylenebis(salicylimine) | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.161 |
| UNII | |
| |
| |
| Properties | |
| C16H16N2O2 | |
| Molar mass | 268.31 |
| Appearance | yellow solid |
| Melting point | 125 to 129 °C (257 to 264 °F; 398 to 402 K) |
| organic polar solvents | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
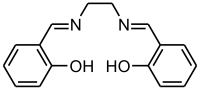
Salen is the common name of a popular chelating ligand used in coordination chemistry and homogeneous catalysis, whose systematic name is N,N'-bis(salicylidene)ethylenediamine. The name salen is a contraction for salicylaldehyde and ethylenediamine.
The metal-free compound, sometimes denoted H
2(salen),[1] is a bright yellow micaceous solid with formula C
16H
16N
2O
2, soluble in polar organic solvents. Its molecular structure can be described as two salicylimine groups (C
6H
4)(OH)(CH)=N– connected by an ethylene bridge –CH
2CH
2–.
Salen complexes normally contain the conjugate base of the metal-free compound, namely the divalent anion salen2−
that results from loss of the protons from the two hydroxyl groupls –OH. This dianion is usually denoted simply as "(salen)" in formulas. It usually acts as a tetradentate ligand for the central metal atom, with coordination bonds to the oxygen and nitrogen atoms. Thus for example the cobalt complex "Co(salen)" or salcomine is an electrically neutral molecule with one cobalt(II) cation Co2+
and one salen anion ligand salen2−
.[1]
The name "salen" is also used generically for similar tetradentate ligands with the same chemical environment around the chelating site. As a ligand, the salen anion also resembles other tetradentate macrocyclicligands, such as porphyrinate, corrin, bis(dimethylglyoximate), and some Schiff bases.
History
Salen and its cobalt complex were first prepared by Tsumaki around 1933.[2][1]
Preparation
H2salen is commercially available. It is often generated in situ followed by the addition of the metal salt, but the ligand is also easily prepared as a pure organic compound by the condensation of ethylenediamine and salicylaldehyde.[3][4]
The reactants are in principle in equilibrium with the product, and water may be removed via an added drying agent or by azeotropic distillation.[5] In practice, the reaction of the salicylaldehyde with the amine in alcoholic solvent usually goes to completion.
Related ligands
Substituted salen
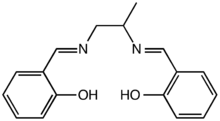
The general class of "salen ligands" includes compounds formally derived from salen by substituting various functional groups on the salicylaldehyde or the ethylene bridge. For examṕle, the salpn ligand, prepared from 1,2-diaminopropane instead of ethylenediamine, is used as a metal deactivation additive in fuels.[6]
Bulky groups near the coordination site may be added to enhance the catalytic activity of the metal complexes and prevent its dimerization. Salen ligands derived from 3,5-di-tert-butylsalicylaldehyde fulfill these roles, and also increase the solubility of the complexes in non-polar solvents like pentane.
Chiral "salen" ligands may be created by proper substitution of the diamine backbone, the phenyl ring, or both.[5] An example is the ligand obtained by condensation of the C2-symmetric trans-1,2-diaminocyclohexane with 3,5-di-tert-butylsalicylaldehdye. Chiral ligands may be used in asymmetric synthesis reactions, such as the Jacobsen epoxidation:[7][8]
.png)
Salen-type ligands
The name "salen" or "salen type" may be used for other ligands that have similar environment around the chelating site, namely two acidic hydoxyls and two Schiff base (aryl-imine) groups. These include the ligands abbreviated as salph, from the condensation of 1,2-phenylenediamine and salicyaldehyde, and salqu, from the condensation of salicylaldehyde and 2-quinoxalinol.[9]

Salen-type ligands may be derived from non-aromatic precursors, such as the condensation of ketoaldehyde equivalents and diamines. The canonical representative is acacen, (more properly H2acacen), obtained by the condensation of acetylacetone and ethylenediamine.[10][11]
Related ligands
The salan and salalen ligands are similar in structure to salen ligands, but have one or two saturated nitrogen-aril bonds (amines rather than imines). They tend to be less rigid and more electron rich at the metal center than the corresponding salen complexes.[12][13][14][15] Salans can be synthesized by the alkylation of an appropriate amine with a phenolic alkyl halide.
The "half-salen" ligands have only one salicylimine group, and therefore are bidentate rather than tetradentate. They are prepared from a salicylaldehyde and a monoamine.[16]
References
- 1 2 3 Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. (1933). "Tricyclische orthokondensierte Nebenvalenzringe" [Tricyclic ortho-condensed outer valence rings]. Liebigs Ann. Chem. 503: 84–130. doi:10.1002/jlac.19335030106.
- ↑ Tsumaki, T. (1938). "Nebenvalenzringverbindungen. IV. Über einige innerkomplexe Kobaltsalze der Oxyaldimine". Bull. Chem. Soc. Jap. (in German). 13 (2): 252–260. doi:10.1246/bcsj.13.252.
- ↑ Diehl, Harvey; Hach, Clifford C. (1950). "Bis(N,N′-Disalicylalethylenediamine)-μ-Aquodicobalt(II)". Inorg. Synth. 3: 196–201. doi:10.1002/9780470132340.ch53. ISBN 978-0-470-13234-0.
- ↑ Harvey Diehl; Clifford C. Hach (1950). "Bis(N,N'-Disalicylalethylenediamine)-μ-Aquodicobalt(II)". Inorg. Synth. Inorganic Syntheses. 3: 196–201. doi:10.1002/9780470132340.ch53. ISBN 978-0-470-13234-0.
- 1 2 Cozzi, Pier Giorgio (2004). "Metal-Salen Schiff base complexes in catalysis: Practical aspects". Chem. Soc. Rev. 33 (7): 410–21. doi:10.1039/B307853C. PMID 15354222.
- ↑ Dabelstein, W.; Reglitzky A.; Schutze A.; Reders, K., "Automotive Fuels", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a16_719.pub2
- ↑ Larrow, J. F.; Jacobsen, E. N. (2004). "(R,R)-N,N'-Bis(3,5-Di-tert-Butylsalicylidene)-1,2-Cyclohexanediamino Manganese(III) Chloride, A Highly Enantioselective Epoxidation Catalyst". Organic Syntheses. ; Collective Volume, 10, p. 96
- ↑ Yoon, TP; Jacobsen, EN (2003). "Privileged Chiral Catalysts". Science. 299 (5613): 1691–1693. doi:10.1126/science.1083622. PMID 12637734.
- ↑ Wu, Xianghong, Gorden, A. V. E., (2009). "2-Quinoxalinol Salen Copper Complexes for Oxidation of Aryl Methylenes". Eur. J. Org. Chem. 4 (4): 503–509. doi:10.1002/ejoc.200800928.
- 1 2 Weber, Birgit; Jäger, Ernst-G. (2009). "Structure and Magnetic Properties of Iron(II/III) Complexes with N
2O2–
2-Coordinating Schiff Base-Like Ligands". Eur. J. Inorg. Chem.: 455. doi:10.1002/ejic.200990003. - ↑ Riley, Dennis P.; Busch, Daryle H. (1978). "Macrocyclic Tetraazatetraenato Ligands and their Metal Complexes". Inorg. Synth. 18: 36. doi:10.1002/9780470132494.ch7.
- ↑ Atwood, David A.; Remington, Michael P.; Rutherford, Drew (1996). "Use of the Salan Ligands to Form Bimetallic Aluminum Complexes". Organometallics. 15 (22): 4763. doi:10.1021/om960505r.
- ↑ Berkessel, Albrecht; Brandenburg, Marc; Leitterstorf, Eva; Frey, Julia; Lex, Johann; Schäfer, Mathias (2007). "A Practical and Versatile Access to Dihydrosalen (Salalen) Ligands: Highly Enantioselective TitaniumIn Situ Catalysts for Asymmetric Epoxidation with Aqueous Hydrogen Peroxide". Adv. Synth. Catal. 349 (14–15): 2385. doi:10.1002/adsc.200700221.
- ↑ Atwood, David A.; Remington, Michael P.; Rutherford, Drew (1996). "Use of the Salan Ligands to Form Bimetallic Aluminum Complexes". Organometallics. 15 (22): 4763. doi:10.1021/om960505r.
- ↑ Berkessel, Albrecht; Brandenburg, Marc; Leitterstorf, Eva; Frey, Julia; Lex, Johann; Schäfer, Mathias (2007). "A Practical and Versatile Access to Dihydrosalen (Salalen) Ligands: Highly Enantioselective Titanium In Situ Catalysts for Asymmetric Epoxidation with Aqueous Hydrogen Peroxide". Adv. Synth. Catal. 349 (14–15): 2385. doi:10.1002/adsc.200700221.
- ↑ Xuan Pang, Ranlong Duan, Xiang Li, Zhiqiang Sun, Han Zhang, Xianhong Wang and Xuesi Chen (2014): "Synthesis and characterization of half-salen complexes and their application in the polymerization of lactide and ε-caprolactone" Polymer Chemistry, volume 5, issue 23, pages 6857-6864. doi:10.1039/C4PY00734D
See also
- Metal salen complexes
- Bisthiosemicarbazones - a structurally related class of C2-symmetric imine based ligands
