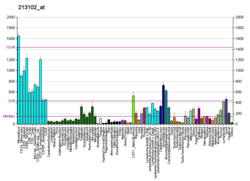
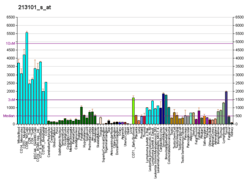
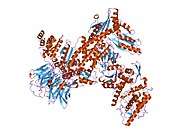
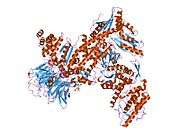
ACTR3
Actin-related protein 3 is a protein that in humans is encoded by the ACTR3 gene.[5]
Function
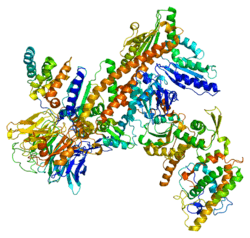
The specific function of this gene has not yet been determined; however, the protein it encodes is known to be a major constituent of the ARP2/3 complex. This complex is located at the cell surface and is essential to cell shape and motility through lamellipodial actin assembly and protrusion.[6]
Interactions
References
- 1 2 3 GRCh38: Ensembl release 89: ENSG00000115091 - Ensembl, May 2017
- 1 2 3 GRCm38: Ensembl release 89: ENSMUSG00000026341 - Ensembl, May 2017
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Welch MD, DePace AH, Verma S, Iwamatsu A, Mitchison TJ (Jul 1997). "The human Arp2/3 complex is composed of evolutionarily conserved subunits and is localized to cellular regions of dynamic actin filament assembly". The Journal of Cell Biology. 138 (2): 375–84. doi:10.1083/jcb.138.2.375. PMC 2138188. PMID 9230079.
- ↑ "Entrez Gene: ACTR3 ARP3 actin-related protein 3 homolog (yeast)".
- ↑ Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT (Oct 2000). "Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex". The Journal of Cell Biology. 151 (1): 29–40. doi:10.1083/jcb.151.1.29. PMC 2189811. PMID 11018051.
- ↑ Di Ciano C, Nie Z, Szászi K, Lewis A, Uruno T, Zhan X, Rotstein OD, Mak A, Kapus A (Sep 2002). "Osmotic stress-induced remodeling of the cortical cytoskeleton". American Journal of Physiology. Cell Physiology. 283 (3): C850–65. doi:10.1152/ajpcell.00018.2002. PMID 12176742.
Further reading
- Welch MD, Iwamatsu A, Mitchison TJ (Jan 1997). "Actin polymerization is induced by Arp2/3 protein complex at the surface of Listeria monocytogenes". Nature. 385 (6613): 265–9. doi:10.1038/385265a0. PMID 9000076.
- Winter D, Podtelejnikov AV, Mann M, Li R (Jul 1997). "The complex containing actin-related proteins Arp2 and Arp3 is required for the motility and integrity of yeast actin patches". Current Biology. 7 (7): 519–29. doi:10.1016/S0960-9822(06)00223-5. PMID 9210376.
- Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, Burlingame AL, Hsuan JJ, Segal AW (Nov 1997). "Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins". The Biochemical Journal. 328 ( Pt 1) (1): 105–12. PMC 1218893. PMID 9359840.
- Ma L, Rohatgi R, Kirschner MW (Dec 1998). "The Arp2/3 complex mediates actin polymerization induced by the small GTP-binding protein Cdc42". Proceedings of the National Academy of Sciences of the United States of America. 95 (26): 15362–7. doi:10.1073/pnas.95.26.15362. PMC 28048. PMID 9860974.
- Machesky LM, Insall RH (1999). "Scar1 and the related Wiskott-Aldrich syndrome protein, WASP, regulate the actin cytoskeleton through the Arp2/3 complex". Current Biology. 8 (25): 1347–56. doi:10.1016/S0960-9822(98)00015-3. PMID 9889097.
- Suetsugu S, Miki H, Takenawa T (Jun 1999). "Identification of two human WAVE/SCAR homologues as general actin regulatory molecules which associate with the Arp2/3 complex". Biochemical and Biophysical Research Communications. 260 (1): 296–302. doi:10.1006/bbrc.1999.0894. PMID 10381382.
- May RC, Hall ME, Higgs HN, Pollard TD, Chakraborty T, Wehland J, Machesky LM, Sechi AS (Jul 1999). "The Arp2/3 complex is essential for the actin-based motility of Listeria monocytogenes". Current Biology. 9 (14): 759–62. doi:10.1016/S0960-9822(99)80337-6. PMID 10421578.
- Loisel TP, Boujemaa R, Pantaloni D, Carlier MF (Oct 1999). "Reconstitution of actin-based motility of Listeria and Shigella using pure proteins". Nature. 401 (6753): 613–6. doi:10.1038/44183. PMID 10524632.
- Higgs HN, Blanchoin L, Pollard TD (Nov 1999). "Influence of the C terminus of Wiskott-Aldrich syndrome protein (WASp) and the Arp2/3 complex on actin polymerization". Biochemistry. 38 (46): 15212–22. doi:10.1021/bi991843. PMID 10563804.
- Carlier MF, Nioche P, Broutin-L'Hermite I, Boujemaa R, Le Clainche C, Egile C, Garbay C, Ducruix A, Sansonetti P, Pantaloni D (Jul 2000). "GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex". The Journal of Biological Chemistry. 275 (29): 21946–52. doi:10.1074/jbc.M000687200. PMID 10781580.
- Jay P, Bergé-Lefranc JL, Massacrier A, Roessler E, Wallis D, Muenke M, Gastaldi M, Taviaux S, Cau P, Berta P (May 2000). "ARP3beta, the gene encoding a new human actin-related protein, is alternatively spliced and predominantly expressed in brain neuronal cells". European Journal of Biochemistry / FEBS. 267 (10): 2921–8. doi:10.1046/j.1432-1327.2000.01306.x. PMID 10806390.
- Weed SA, Karginov AV, Schafer DA, Weaver AM, Kinley AW, Cooper JA, Parsons JT (Oct 2000). "Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex". The Journal of Cell Biology. 151 (1): 29–40. doi:10.1083/jcb.151.1.29. PMC 2189811. PMID 11018051.
- Prehoda KE, Scott JA, Mullins RD, Lim WA (Oct 2000). "Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex". Science. 290 (5492): 801–6. doi:10.1126/science.290.5492.801. PMID 11052943.
- Marchand JB, Kaiser DA, Pollard TD, Higgs HN (Jan 2001). "Interaction of WASP/Scar proteins with actin and vertebrate Arp2/3 complex". Nature Cell Biology. 3 (1): 76–82. doi:10.1038/35050590. PMID 11146629.
- Zhao X, Yang Z, Qian M, Zhu X (Jan 2001). "Interactions among subunits of human Arp2/3 complex: p20-Arc as the hub". Biochemical and Biophysical Research Communications. 280 (2): 513–7. doi:10.1006/bbrc.2000.4151. PMID 11162547.
- Robinson RC, Turbedsky K, Kaiser DA, Marchand JB, Higgs HN, Choe S, Pollard TD (Nov 2001). "Crystal structure of Arp2/3 complex". Science. 294 (5547): 1679–84. doi:10.1126/science.1066333. PMID 11721045.
- Gournier H, Goley ED, Niederstrasser H, Trinh T, Welch MD (Nov 2001). "Reconstitution of human Arp2/3 complex reveals critical roles of individual subunits in complex structure and activity". Molecular Cell. 8 (5): 1041–52. doi:10.1016/S1097-2765(01)00393-8. PMID 11741539.
- Andersen JS, Lyon CE, Fox AH, Leung AK, Lam YW, Steen H, Mann M, Lamond AI (Jan 2002). "Directed proteomic analysis of the human nucleolus". Current Biology. 12 (1): 1–11. doi:10.1016/S0960-9822(01)00650-9. PMID 11790298.
- Kovacs EM, Goodwin M, Ali RG, Paterson AD, Yap AS (Mar 2002). "Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts". Current Biology. 12 (5): 379–82. doi:10.1016/S0960-9822(02)00661-9. PMID 11882288.
External links
- ACTR3 human gene location in the UCSC Genome Browser.
- ACTR3 human gene details in the UCSC Genome Browser.
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.