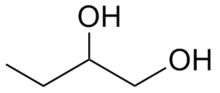
1,2-Butanediol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Butane-1,2-diol | |
| Other names
1,2-Dihydroxybutane α-Butylene glycol | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.008.663 |
| EC Number | 209-527-2 |
PubChem CID |
|
| RTECS number | EK0380000 |
| UNII | |
| |
| |
| Properties[1][2][3] | |
| C4H10O2 | |
| Molar mass | 90.121 g/mol |
| Density | 1.0023 g/cm3 (20 °C) |
| Melting point | −50 °C (−58 °F; 223 K)[note 1] |
| Boiling point | 195 to 196.9 °C (383.0 to 386.4 °F; 468.1 to 470.0 K) (96.5 °C at 10 mmHg) |
| miscible | |
| Solubility | soluble in ethanol, acetone; sparingly soluble in esters and ethers; insoluble in hydrocarbons |
Refractive index (nD) |
1.4378 (20 °C) |
| Viscosity | 7.3 mPa s (20 °C) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH |
−532.8 kJ/mol [4] |
Std enthalpy of combustion (ΔcH |
−2479 kJ/mol |
| Hazards[5] | |
| Safety data sheet | ICSC 0395 |
| Flash point | 90 °C (194 °F; 363 K) |
| Related compounds | |
Related butanediols |
1,3-Butanediol 1,4-Butanediol 2,3-Butanediol |
Related compounds |
Ethylene glycol Propylene glycol 2-Hydroxybutyraldehyde 2-Hydroxybutyric acid α-Ketobutyric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1,2-Butanediol is a vic-diol (glycol) first described by Charles-Adolphe Wurtz in 1859.[6] It is produced industrially as a byproduct of the production of 1,4-butanediol from butadiene,[7] and is also a byproduct of the catalytic hydrocracking of starches and sugars such as sorbitol to ethylene glycol and propylene glycol.[8][note 2] It is used to produce polyester resins and plasticizers,[3][7] and is a potential feedstock for the industrial production of α-ketobutyric acid, a precursor to many amino acids.[9]
Notes
- ↑ The value of −50 °C for the melting point is taken from Ullmann's Encyclopedia of Industrial Chemistry and used by the Hazardous Substances Data Bank and the OECD Screening Information Dataset. Other reported values of the melting point range from −114 °C to −30 °C.
- ↑ The catalytic hydrocracking of starches and sugars has been successfully commercialized by S2G Biochem Inc. in partnership with International Polyol Chemical, Inc., as of 2007. http://www.s2gbiochem.com/technology.html Archived 2012-04-26 at the Wayback Machine.
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-190. ISBN 0-8493-0462-8. .
- ↑ Ullmann's Encyclopedia of Industrial Chemistry, A1, Weinheim: Wiley-VCH, p. VA4 461 .
- 1 2 1,2-Butanediol (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, February 1995 .
- ↑ Moureu, H.; Dode, M. (1937), "Chaleurs de formation de l'oxyde d'ethylene, de l'ethanediol et de quelques homologues", Bull. Soc. Chim. Fr., 4: 637–47 .
- ↑ 1,2-Butanediol, International Chemical Safety Card 0395, Geneva: International Programme on Chemical Safety, March 1996 .
- ↑ Wurtz, A. (1859), Ann. Chim. Phys., 55: 400 .
- 1 2 US 4596886, Hasegawa, Ryuichi & Kohji Hayashi, "Polyester containing impure 1,2-butanediol".
- ↑ US 4966658, Berg, Lloyd, "Recovery of ethylene glycol from butanediol isomers by azeotropic distillation". US 5423955, Berg, Lloyd, "Separation of propylene glycol from 1,2-butanediol by azeotropic distillation".
- ↑ US 5155263, Imanari, Makoto; Hiroshi Iwane & Masashi Suzuki et al., "Process for preparing α-ketobutyric acid".
External links
This article is issued from
Wikipedia.
The text is licensed under Creative Commons - Attribution - Sharealike.
Additional terms may apply for the media files.