pyrolysis
English
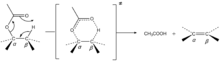
The mechanism of pyrolysis of an ester
Etymology
From the Ancient Greek terms πῦρ (pûr, “fire”) and λύσις (lúsis, “loosing”); synchronically, pyro- + -lysis.
Pronunciation
- (Received Pronunciation)1 IPA(key): /pɪˈɹɒlɪsɪs/
- (Received Pronunciation)2 IPA(key): /paɪˈɹɒlɪsɪs/
Audio (US) (file)
Noun
pyrolysis (countable and uncountable, plural pyrolyses)
- (chemistry, physics) The decomposition of a material or compound due to heat, in the absence of oxygen or other reagents.
- 1972, A. C. Knipe, Chapter 4: Elimination Reactions, B. Capon, C. W. Rees (editors), Organic Reaction Mechanisms 1971, page 143,
- Techniques that have successfully identified ionic intermediates in solution have been applied to gas-phase pyrolyses.
- 1980, J. H. Purnell, Homogeneous Alkane Cracking, William Pryor (editor), Frontiers of Free Radical Chemistry: The route to quantitative description to very high conversion, page 94,
- Twenty years ago our real understanding of the mechanism of alkane pyrolyses was little better than rudimentary.
- 2001, P. T. Williams, R. P. Bottrill, A. J. Brindle, A. M. Cunliffe, The potential of pyrolysis for recycling used tyres, Ravindra K. Dhir, Mukesh C. Limbachiya, Kevin A. Paine (editors), Recycling and Reuse of Used Tyres: Proceedings of the International Symposium, page 187,
- Pyrolysis involves the thermal degradation of the rubber of the tyre to give an oil and gas leaving a residual solid carbon and the steel casing of the tyre.
- 2006, John C. F. Walker, Primary Wood Processing: Principles and Practice, 2nd Edition, Springer, page 541,
- Traditional pyrolysis of wood relies on low temperatures and long processing time to increase the charcoal yield. In contrast, modern or fast pyrolysis uses moderate temperatures (400-500°C) and very short residence times (typically only a few seconds) to maximize the production of liquids (Diebold and Bridgewater, 1997).
- 1972, A. C. Knipe, Chapter 4: Elimination Reactions, B. Capon, C. W. Rees (editors), Organic Reaction Mechanisms 1971, page 143,
Related terms
Translations
decomposition of a material or compound due to heat where there is no oxygen or other reagents
See also
This article is issued from
Wiktionary.
The text is licensed under Creative
Commons - Attribution - Sharealike.
Additional terms may apply for the media files.