Grignard reaction
The Grignard reaction (pronounced /ɡriɲar/) is an organometallic chemical reaction in which alkyl, allyl, vinyl, or aryl-magnesium halides (Grignard reagent) add to a carbonyl group in an aldehyde or ketone.[1][2] This reaction is important for the formation of carbon–carbon bonds.[3][4] The reaction of an organic halide with magnesium is not a Grignard reaction, but provides a Grignard reagent.[5]
| Grignard reaction | |
|---|---|
| Named after | Victor Grignard |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | grignard-reaction |
| RSC ontology ID | RXNO:0000014 |

Grignard reactions and reagents were discovered by and are named after the French chemist François Auguste Victor Grignard (University of Nancy, France), who published it in 1900 and was awarded the 1912 Nobel Prize in Chemistry for this work.[6]
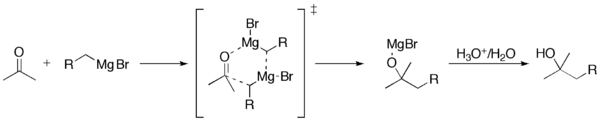
Reaction Mechanism
The carbon attached to magnesium functions as a nucleophile, attacking the electrophilic carbon atom that is present within the polar bond of a carbonyl group. The addition of the Grignard reagent to the carbonyl typically proceeds through a six-membered ring transition state.[7]
See also
| Wikimedia Commons has media related to Grignard reactions. |
- Wittig reaction
- Barbier reaction
- Bodroux-Chichibabin aldehyde synthesis
- Fujimoto-Belleau reaction
- Organolithium reagents
- Sakurai reaction
- Indium mediated allylation
- Alkynylation
References
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- Chapter 19: Carboxylic Acids. Organic Chemistry 4e Carey. mhhe.com
- Shirley, D. A. (1954). "The Synthesis of Ketones from Acid Halides and Organometallic Compounds of Magnesium, Zinc, and Cadmium". Org. React. 8: 28–58.
- Huryn, D. M. (1991). "Carbanions of Alkali and Alkaline Earth Cations: (ii) Selectivity of Carbonyl Addition Reactions". In Trost, B. M.; Fleming, I. (eds.). Comprehensive Organic Synthesis, Volume 1: Additions to C—X π-Bonds, Part 1. Elsevier Science. pp. 49–75. doi:10.1016/B978-0-08-052349-1.00002-0. ISBN 978-0-08-052349-1.
- IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). ISBN 0-9678550-9-8. doi:10.1351/goldbook.
- Grignard, V. (1900). "Sur quelques nouvelles combinaisons organométaliques du magnésium et leur application à des synthèses d'alcools et d'hydrocabures". Compt. Rend. 130: 1322–25.
- Maruyama, K.; Katagiri, T. (1989). "Mechanism of the Grignard reaction". J. Phys. Org. Chem. 2 (3): 205–213. doi:10.1002/poc.610020303.
