Autotroph
An autotroph or primary producer is an organism that produces complex organic compounds (such as carbohydrates, fats, and proteins) using carbon from simple substances such as carbon dioxide,[1] generally using energy from light (photosynthesis) or inorganic chemical reactions (chemosynthesis).[2] Autotrophs do not need a living source of carbon or energy and are the producers in a food chain, such as plants on land or algae in water (in contrast to heterotrophs as consumers of autotrophs or other heterotrophs). Autotrophs can reduce carbon dioxide to make organic compounds for biosynthesis and as stored chemical fuel. Most autotrophs use water as the reducing agent, but some can use other hydrogen compounds such as hydrogen sulfide.
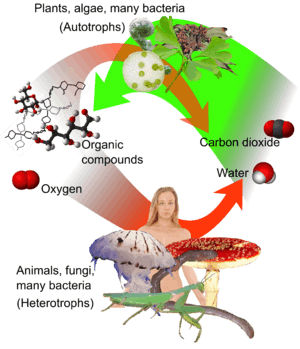
Some autotrophs, such as green plants and algae, are phototrophs, meaning that they convert electromagnetic energy from sunlight into chemical energy in the form of glucose. Others, including methanogens, are chemotrophs, which use organic or inorganic chemical compounds as a source of energy. Most chemoautotrophs are lithotrophs, using inorganic electron donors such as hydrogen sulfide, hydrogen gas, elemental sulfur, ammonium and ferrous oxide as reducing agents and hydrogen sources for biosynthesis and chemical energy release. Autotrophs use a portion of the ATP produced during photosynthesis or the oxidation of chemical compounds to reduce NADP+ to NADPH to form organic compounds.[3]
History
The Greek term autotroph was coined by the German botanist Albert Bernhard Frank in 1892.[4] It stems from the ancient Greek word τροφή (trophḗ), meaning "nourishment" or "food". The first autotrophic organism developed about 2 billion years ago.[5] Photoautotrophs evolved from heterotrophic bacteria by developing photosynthesis. The earliest photosynthetic bacteria used hydrogen sulphide. Due to the scarcity of hydrogen sulphide, some photosynthetic bacteria evolved to use water in photosynthesis, leading to cyanobacteria.[6]
Variants
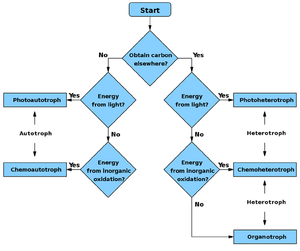
Some organisms rely on organic compounds as a source of carbon, but are able to use light or inorganic compounds as a source of energy. Such organisms are not defined as autotrophic, but rather as heterotrophic. An organism that obtains carbon from organic compounds but obtains energy from light is called a photoheterotroph, while an organism that obtains carbon from organic compounds and energy from the oxidation of inorganic compounds is termed a chemolithoheterotroph.
Evidence suggests that some fungi may also obtain energy from ionizing radiation: Such radiotrophic fungi were found growing inside a reactor of the Chernobyl nuclear power plant.[7]
Ecology

Autotrophs are fundamental to the food chains of all ecosystems in the world. They take energy from the environment in the form of sunlight or inorganic chemicals and use it to create fuel molecules such as carbohydrates. This mechanism is called primary production. Other organisms, called heterotrophs, take in autotrophs as food to carry out functions necessary for their life. Thus, heterotrophs – all animals, almost all fungi, as well as most bacteria and protozoa – depend on autotrophs, or primary producers, for the raw materials and fuel they need. Heterotrophs obtain energy by breaking down carbohydrates or oxidizing organic molecules (carbohydrates, fats, and proteins) obtained in food.[8] Carnivorous organisms rely on autotrophs indirectly, as the nutrients obtained from their heterotrophic prey come from autotrophs they have consumed.
Most ecosystems are supported by the autotrophic primary production of plants and cyanobacteria that capture photons initially released by the sun. Plants can only use a fraction (approximately 1%) of this energy for photosynthesis.[9] The process of photosynthesis splits a water molecule (H2O), releasing oxygen (O2) into the atmosphere, and reducing carbon dioxide (CO2) to release the hydrogen atoms that fuel the metabolic process of primary production. Plants convert and store the energy of the photon into the chemical bonds of simple sugars during photosynthesis. These plant sugars are polymerized for storage as long-chain carbohydrates, including other sugars, starch, and cellulose; glucose is also used to make fats and proteins. When autotrophs are eaten by heterotrophs, i.e., consumers such as animals, the carbohydrates, fats, and proteins contained in them become energy sources for the heterotrophs.[10] Proteins can be made using nitrates, sulfates, and phosphates in the soil.[11][12]
See also
- Electrolithoautotroph
- Electrotroph
- Heterotrophic nutrition
- Organotroph
- Primary nutritional groups
References
- Morris, J. et al. (2019). "Biology: How Life Works", 3rd edition, W. H. Freeman. ISBN 978-1319017637
- Chang, Kenneth (12 September 2016). "Visions of Life on Mars in Earth's Depths". The New York Times. Retrieved 12 September 2016.
- Mauseth, James D. (2008). Botany: An Introduction to Plant Biology (4 ed.). Jones & Bartlett Publishers. p. 252. ISBN 978-0-7637-5345-0.
- Frank, Albert Bernard (1892–93). Lehrbuch der Botanik. Leipzig: W. Engelmann.
- "Bacteria Knowledge". eni school energy & environment. Retrieved 3 May 2019.
- Townsend, Rich (13 October 2019). "The Evolution of Autotrophs". University of Wisconsin-Madison Department of Astronomy. Retrieved 3 May 2019.
- Melville, Kate (23 May 2007). "Chernobyl fungus feeds on radiation". Archived from the original on 4 February 2009. Retrieved 18 February 2009.
- Schmidt-Rohr, K. (2015). "Why Combustions Are Always Exothermic, Yielding About 418 kJ per Mole of O2", J. Chem. Educ. 92: 2094-2099. http://dx.doi.org/10.1021/acs.jchemed.5b00333
- Schurr, Sam H. Energy, Economic Growth, and the Environment. New York. ISBN 9781617260209. OCLC 868970980.
- Beckett, Brian S. (1981). Illustrated Human and Social Biology. Oxford University Press. p. 38. ISBN 978-0-19-914065-7.
- Odum, E.P.; Barrett, G.W. (2005). Fundamentals of ecology. Brooks Cole. p. 598. ISBN 978-0-534-42066-6.
- Smith, Gilbert M. (2007). A Textbook of General Botany. Read Books. p. 148. ISBN 978-1-4067-7315-6.
| Look up autotroph in Wiktionary, the free dictionary. |