Nitrate
Nitrate is a polyatomic ion with the molecular formula NO−
3. Organic compounds that contain the nitrate ester as a functional group (RONO2) are also called nitrates. Nitrates are common components of fertilizers and explosives.[1] Almost all nitrate salts are soluble in water. A common example of an inorganic nitrate salt is potassium nitrate (saltpeter).
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Nitrate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
| NO− 3 | |
| Molar mass | 62.004 g·mol−1 |
| Conjugate acid | Nitric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure
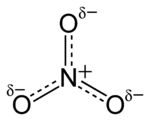
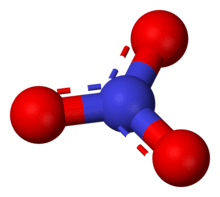
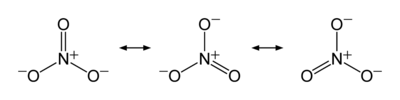
The anion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a −2⁄3 charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures:
Dietary nitrates
A rich source of inorganic nitrate in the human diets come from leafy green foods, such as spinach and arugula. NO−
3 (inorganic nitrate) is the viable active component within beetroot juice and other vegetables. Drinking water is also a dietary source.[2]
Dietary nitrate supplementation delivers positive results when testing endurance exercise performance.[3]
Ingestion of large doses of nitrate either in the form of pure sodium nitrate or beetroot juice in young healthy individuals rapidly increases plasma nitrate concentration about 2-3 fold, and this elevated nitrate concentration can be maintained for at least 2 weeks. Increased plasma nitrate stimulates the production of nitric oxide. Nitric oxide is important physiological signalling molecule that is used in, among other things, regulation of muscle blood flow and mitochondrial respiration.[4]
Cured meats
Nitrite consumption is primarily determined by the amount of processed meats eaten, and the concentration of nitrates in these meats. Although nitrites are the nitrogen compound chiefly used in meat curing, nitrates are used as well. Nitrates lead to the formation of nitrosamines.[5] The production of carcinogenic nitrosamines may be inhibited by the use of the antioxidants vitamin C and the alpha-tocopherol form of vitamin E during curing.[6]
Anti-hypertensive diets, such as the DASH diet, typically contain high levels of nitrates, which are first reduced to nitrite in the saliva, as detected in saliva testing, prior to forming nitric oxide.[2]
Occurrence and production
Nitrate salts are found naturally on earth as large deposits, particularly of nitratine, a major source of sodium nitrate.
Nitrates are produced by a number of species of nitrifying bacteria, and the nitrate compounds for gunpowder (see this topic for more) were historically produced, in the absence of mineral nitrate sources, by means of various fermentation processes using urine and dung.
As a byproduct of lightning strikes in earth's nitrogen-oxygen rich atmosphere, nitric acid is produced when nitrogen dioxide reacts with water vapor.
Nitrates are produced industrially from nitric acid.[1]
Uses

Nitrates are mainly produced for use as fertilizers in agriculture because of their high solubility and biodegradability. The main nitrate fertilizers are ammonium, sodium, potassium, calcium, and magnesium salts. Several million kilograms are produced annually for this purpose.[1]
The second major application of nitrates is as oxidizing agents, most notably in explosives where the rapid oxidation of carbon compounds liberates large volumes of gases (see gunpowder for an example). Sodium nitrate is used to remove air bubbles from molten glass and some ceramics. Mixtures of the molten salt are used to harden some metals.[1]
Detection
Almost all methods for detection of nitrate rely on its conversion to nitrite followed by nitrite-specific tests. The reduction of nitrate to nitrite is effected by copper-cadmium material. The sample is introduced with a flow injection analyzer, and the resulting nitrite-containing effluent is then combined with a reagent for colorimetric or electrochemical detection. The most popular of these assays is the Griess test, whereby nitrite is converted to an deeply colored azo dye, suited for UV-vis spectroscopic analysis. The method exploits the reactivity of nitrous acid derived from acidification of nitrite. Nitrous acid selectively reacts with aromatic amines to give diazonium salts, which in turn couple with a second reagent to give the azo dye. The detection limit is 0.02 to 2 μM.[7] Methods have been highly adapted to biological samples.[8]
Safety
The acute toxicity of nitrate is low. "Substantial disagreement" exists about the long-term risks of nitrate exposure. The two areas of possible concern are that (i) nitrate could be a precursor to nitrite in the lower gut, and nitrite is a precursor to nitrosamines, which are implicated in carcinogenesis, and (ii) nitrate is implicated in methemoglobinemia, a disorder of red blood cells hemoglobin.[9][10]
Methemoglobinemia
Nitrates do not affect infants and pregnant women.[11][12] Blue baby syndrome is caused by a number of other factors such as gastric upset, such as diarrheal infection, protein intolerance, heavy metal toxicity etc., with nitrates playing a minor role.[13]
Drinking water standards
Through the Safe Drinking Water Act, the United States Environmental Protection Agency has set a maximum contaminant level of 10 mg/L or 10 ppm of nitrates in drinking water.[14]
It should be added that an acceptable daily intake (ADI) for nitrate ions was established in the range of 0–3.7 mg (kg body weight)−1 day−1 by the Joint FAO/WHO Expert Committee on Food additives (JEFCA).[15]
Aquatic toxicity
In freshwater or estuarine systems close to land, nitrate can reach concentrations that are lethal to fish. While nitrate is much less toxic than ammonia,[16] levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species.[17] Nitrate toxicity remains the subject of debate.[18]
In most cases of excess nitrate concentrations in aquatic systems, the primary source is surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. The resulting eutrophication and algae blooms result in anoxia and dead zones. As a consequence, as nitrate forms a component of total dissolved solids, they are widely used as an indicator of water quality.
Domestic animal feed
Symptoms of nitrate poisoning in domestic animals include increased heart rate and respiration; in advanced cases blood and tissue may turn a blue or brown color. Feed can be tested for nitrate; treatment consists of supplementing or substituting existing supplies with lower nitrate material. Safe levels of nitrate for various types of livestock are as follows:[19]
| Category | %NO3 | %NO3–N | %KNO3 | Effects |
|---|---|---|---|---|
| 1 | < 0.5 | < 0.12 | < 0.81 | Generally safe for beef cattle and sheep |
| 2 | 0.5–1.0 | 0.12–0.23 | 0.81–1.63 | Caution: some subclinical symptoms may appear in pregnant horses, sheep and beef cattle |
| 3 | 1.0 | 0.23 | 1.63 | High nitrate problems: death losses and abortions can occur in beef cattle and sheep |
| 4 | < 1.23 | < 0.28 | < 2.00 | Maximum safe level for horses. Do not feed high nitrate forages to pregnant mares |
The values above are on a dry (moisture-free) basis.
Salts and covalent derivatives
Nitrate formation with elements of the periodic table.
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
C | NO− 3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
See also
- Ammonium
- f-ratio
- Nitrification
- Nitratine
- Peroxynitrate
- Sodium nitrate
References
- Laue W, Thiemann M, Scheibler E, Wiegand KW (2006). "Nitrates and Nitrites". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_265.
- Hord NG, Tang Y, Bryan NS (July 2009). "Food sources of nitrates and nitrites: the physiologic context for potential health benefits". The American Journal of Clinical Nutrition. 90 (1): 1–10. doi:10.3945/ajcn.2008.27131. PMID 19439460.
- McMahon NF, Leveritt MD, Pavey TG (April 2017). "The Effect of Dietary Nitrate Supplementation on Endurance Exercise Performance in Healthy Adults: A Systematic Review and Meta-Analysis" (PDF). Sports Medicine (Auckland, N.Z.). 47 (4): 735–756. doi:10.1007/s40279-016-0617-7. PMID 27600147.
- Maughan, Ronald J (2013). Food, Nutrition and Sports Performance III. New York: Taylor & Francis. p. 63. ISBN 978-0-415-62792-4.
- Bingham SA, Hughes R, Cross AJ (November 2002). "Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response". The Journal of Nutrition. 132 (11 Suppl): 3522S–3525S. doi:10.1093/jn/132.11.3522S. PMID 12421881.
- Parthasarathy DK, Bryan NS (November 2012). "Sodium nitrite: the "cure" for nitric oxide insufficiency". Meat Science. 92 (3): 274–9. doi:10.1016/j.meatsci.2012.03.001. PMID 22464105.
- Moorcroft, M.; Davis, J.; Compton, R. G. (2001). "Detection and determination of nitrate and nitrite: A review". Talanta. 54 (5): 785–803. doi:10.1016/S0039-9140(01)00323-X. PMID 18968301.
- Ellis, Graham; Adatia, Ian; Yazdanpanah, Mehrdad; Makela, Sinikka K. (1998). "Nitrite and Nitrate Analyses: A Clinical Biochemistry Perspective". Clinical Biochemistry. 31 (4): 195–220. doi:10.1016/S0009-9120(98)00015-0. PMID 9646943.
- Powlson, David S.; Addiscott, Tom M.; Benjamin, Nigel; Cassman, Ken G.; De Kok, Theo M.; Van Grinsven, Hans; l'Hirondel, Jean-Louis; Avery, Alex A.; Van Kessel, Chris (2008). "When Does Nitrate Become a Risk for Humans?". Journal of Environmental Quality. 37 (2): 291–5. doi:10.2134/jeq2007.0177. PMID 18268290.
- "Nitrate and Nitrite Poisoning: Introduction". The Merck Veterinary Manual. Retrieved 2008-12-27.
- Addiscott, T.M.; Benjamin, N. (2006). "Nitrate and human health". Soil Use and Management. 20 (2): 98–104. doi:10.1111/j.1475-2743.2004.tb00344.x.
- A. A. Avery: Infant Methemoglobinemia - Reexamining the Role of Drinking Water Nitrates, Environmental Health Perspectives, Volume 107, Number 7, July 1999
- Manassaram DM, Backer LC, Messing R, Fleming LE, Luke B, Monteilh CP (October 2010). "Nitrates in drinking water and methemoglobin levels in pregnancy: a longitudinal study". Environmental Health. 9 (1): 60. doi:10.1186/1476-069x-9-60. PMC 2967503. PMID 20946657.
- "4. What are EPA's drinking water regulations for nitrate?". Ground Water & Drinking Water. Retrieved 2018-11-13.
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. (2017). "Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate". Journal of Hazardous Materials. 324 (Pt B): 762–772. doi:10.1016/j.jhazmat.2016.11.055. PMID 27894754.
- Romano N, Zeng C (September 2007). "Acute toxicity of sodium nitrate, potassium nitrate, and potassium chloride and their effects on the hemolymph composition and gill structure of early juvenile blue swimmer crabs(Portunus pelagicus Linnaeus, 1758) (Decapoda, Brachyura, Portunidae)". Environmental Toxicology and Chemistry. 26 (9): 1955–62. doi:10.1897/07-144r.1. PMID 17705664.
- Sharpe, Shirlie. "Nitrates in the Aquarium". About.com. Retrieved October 30, 2013.
- Romano N, Zeng C (December 2007). "Effects of potassium on nitrate mediated alterations of osmoregulation in marine crabs". Aquatic Toxicology. 85 (3): 202–8. doi:10.1016/j.aquatox.2007.09.004. PMID 17942166.
- "Nitrate Risk in Forage Crops - Frequently Asked Questions". Agriculture and Rural Development. Government of Alberta. Retrieved October 30, 2013.