Wurtz reaction
The Wurtz reaction, named after Charles Adolphe Wurtz, is a coupling reaction in organic chemistry, organometallic chemistry and recently inorganic main group polymers, whereby two alkyl halides are reacted with sodium metal in dry ether solution to form a higher alkane. In this reaction alkyl halides are treated with sodium metal in dry ethereal (free from moisture) solution to produce higher alkanes and it is also used for the preparation of higher alkanes containing even number of carbon atoms.
- 2 R–X + 2 Na → R–R + 2 Na+X−
| Wurtz reaction | |
|---|---|
| Named after | Charles Adolphe Wurtz |
| Reaction type | Coupling reaction |
| Identifiers | |
| Organic Chemistry Portal | wurtz-reaction |
| RSC ontology ID | RXNO:0000074 |
Other metals have also been used to effect the Wurtz coupling, among them silver, zinc, iron, activated copper, indium and a mixture of manganese and copper chloride.[1] The related reaction dealing with aryl halides is called the Wurtz–Fittig reaction. This can be explained by the formation of free radical intermediate and its subsequent disproportionation to give alkene. The Wurtz reaction occurs through a free radical mechanism that makes possible side reactions producing alkene products.
Mechanism
The reaction consists of a halogen-metal exchange involving the radical species R• (in a similar fashion to the formation of a Grignard reagent) with carbon–carbon bond formation occurring in a nucleophilic substitution reaction.
One electron from the metal is transferred to the halogen to produce a metal halide and an alkyl radical.
- R–X + M → R• + M+X−
The alkyl radical then accepts an electron from another metal atom to form an alkyl anion. This intermediate has been isolated in several cases.
- R• + M → R−M+
The nucleophilic carbon of the alkyl anion then displaces the halide in an SN2 reaction, forming a new carbon-carbon covalent bond.
- R−M+ + R–X → R–R + M+X−
Examples and reaction conditions
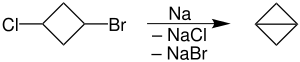
Due to several limitations this reaction is seldom used. For example, it is intolerant of a range of functional groups. Wurtz coupling is, however, useful in closing small, especially three-membered, rings. Bicyclobutane was prepared this way from 1-bromo-3-chlorocyclobutane in 95% yield. The reaction is conducted in refluxing dioxane, at which temperature, the sodium is liquid. This reaction has poor yield which is a consequence of multiple product formation. In the case of (1,3), (1,4), (1,5), (1,6) dihalides, it leads to formation of cyclic products. In vicinal dihalides, it forms alkenes, whereas in geminal dihalides, it forms alkynes.[2]
Limitations
The Wurtz reaction is seldom used because of side reactions.[3] It has limited use to the synthesis of symmetric alkanes. If two dissimilar alkyl halides are taken as reactants, then the product is a mixture of alkanes that is often difficult to separate by fractional distillation as the differences between the boiling points of the products are typically very low. Methane can not be obtained by this method. This type of reaction fails in case of tertiary halides. Also, since the reaction involves free radical species, a side reaction occurs to produce an alkene. This side reaction becomes more significant when the alkyl halides are bulky at the halogen-attached carbon atom.
See also
References
- March Advanced Organic Chemistry 4th edition p. 535
- Gary M. Lampman and James C. Aumiller "Bicyclo[1.1.0]butane" Organic Syntheses, 1971, volume 51, pp 55-9. doi:10.15227/orgsyn.051.0055 Aditya Krishna "Dihalides Quartz"
- March Advanced Organic Chemistry 7th edition p. 512
- Adolphe Wurtz (1855). "Sur une nouvelle classe de radicaux organiques". Annales de chimie et de physique. 44: 275–312.
- Adolphe Wurtz (1855). "Ueber eine neue Klasse organischer Radicale". Annalen der Chemie und Pharmacie. 96 (3): 364–375. doi:10.1002/jlac.18550960310.
- Organic-chemistry.org
- Organic Chemistry, by Morrison and Boyd
- Organic Chemistry, by Graham Solomons and Craig Fryhle, Wiley Publications