Trifarotene
Trifarotene, sold under the brand name Aklief, is a drug for the topical treatment of acne vulgaris in those nine years of age and older.[1] It is a retinoid;[2] more specifically, it is a fourth generation selective retinoic acid receptor (RAR)-γ agonist.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Aklief |
| Other names | CD5789 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Routes of administration | Topical |
| Drug class | Skin and mucous membrane agents |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
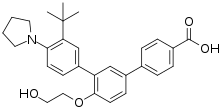
| Formula | C29H33NO4 |
| Molar mass | 459.586 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It was approved for medical use in the United States in 2019,[1][4][5] but is not approved in the European Union as of June 2020.[6]
References
- "Drug Trials Snapshots: Aklief". U.S. Food and Drug Administration (FDA). 11 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.

- Trifarotene Monograph
- Scott, Lesley J. (2019). "Trifarotene: First Approval". Drugs. 79 (17): 1905–1909. doi:10.1007/s40265-019-01218-6. ISSN 0012-6667.
- "Aklief (trifarotene) FDA Approval History". Drugs.com. 7 October 2019. Retrieved 19 November 2019.
- "Drug Approval Package: Aklief". U.S. Food and Drug Administration (FDA). 21 October 2019. Archived from the original on 19 November 2019. Retrieved 18 November 2019.
- "Trifarotene". European Medicines Agency. Retrieved 17 June 2020.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.