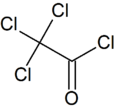
Trichloroacetyl chloride
Trichloroacetyl chloride is the acyl chloride of trichloroacetic acid. It can be formed by reacting chlorine with acetyl chloride or acetaldehyde in the presence of activated charcoal. It is used in the manufacture of pharmaceuticals and plant protection compounds.[3]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloroacetyl chloride | |||
| Other names
2,2,2-Trichloroacetyl chloride | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.000.843 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2Cl4O | |||
| Molar mass | 181.832 g/mol | ||
| Density | 1.62 g/cm3 at 20 °C | ||
| Boiling point | 117.9 °C (244.2 °F; 391.0 K) | ||
| Solubility | miscible with diethyl ether[1] | ||
| Thermochemistry | |||
Std enthalpy of formation (ΔfH⦵298) |
-280.0 kJ•mol−1[2] | ||
| Hazards | |||
| Safety data sheet | Oxford MSDS | ||
EU classification (DSD) (outdated) |
Toxic (T); Corrosive (C) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3–536, ISBN 0-8493-0594-2
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 5–29, ISBN 0-8493-0594-2
- US Patent No. 5,659,078 to Ebmeyer et al., "Process for the preparation of trichloroacetyl chloride," issued August 19, 1997 (as reproduced by freepatentsonline.com and retrieved on October 23, 2007).
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.