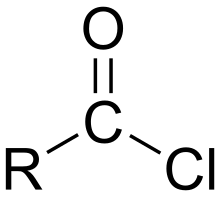
Acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group -COCl. Their formula is usually written RCOCl, where R is a side chain. They are reactive derivatives of carboxylic acids. A specific example of an acyl chloride is acetyl chloride, CH3COCl. Acyl chlorides are the most important subset of acyl halides.
Nomenclature
Where the acyl chloride moiety takes priority, acyl chlorides are named by taking the name of the parent carboxylic acid, and substituting -yl chloride for -ic acid. Thus:
- acetyl chloride CH3COCl
- benzoyl chloride C6H5COCl
When other functional groups take priority, acyl chlorides are considered prefixes — chlorocarbonyl-:[1]
- (chlorocarbonyl)acetic acid ClOCCH2COOH
Properties
Lacking the ability to form hydrogen bonds, acid chlorides have lower boiling and melting points than similar carboxylic acids. For example, acetic acid boils at 118 °C, whereas acetyl chloride boils at 51 °C. Like most carbonyl compounds, infrared spectroscopy reveals a band near 1750 cm−1.
The simplest stable acyl chloride is ethanoyl chloride or acetyl chloride; methanoyl chloride (formyl chloride) is not stable at room temperature, although it can be prepared at –60 °C or below.[2][3] Acyl chloride is not soluble in water. Instead, it decomposes in water.
Synthesis
Industrial routes
The industrial route to acetyl chloride involves the reaction of acetic anhydride with hydrogen chloride:[4]
- (CH3CO)2O + HCl → CH3COCl + CH3CO2H
Propionyl chloride is produced by chlorination of propionic acid with phosgene:[5]
- CH3CH2CO2H + COCl2 → CH3CH2COCl + HCl + CO2
Benzoyl chloride is produced by the partial hydrolysis of benzotrichloride:[6]
- C6H5CCl3 + H2O → C6H5C(O)Cl + 2 HCl
Laboratory methods
In the laboratory, acyl chlorides are generally prepared in the same manner as alkyl chlorides, by replacing the corresponding hydroxy substituents with chlorides. Thus, carboxylic acids are treated with thionyl chloride (SOCl2),[7] phosphorus trichloride (PCl3),[8] or phosphorus pentachloride (PCl5):[9][10]
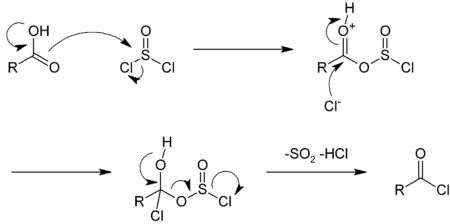
- RCOOH + SOCl2 → RCOCl + SO2 + HCl
- 3 RCOOH + PCl3 → 3 RCOCl + H3PO3
- RCOOH + PCl5 → RCOCl + POCl3 + HCl
The reaction with thionyl chloride may be catalyzed by dimethylformamide.[11] In this reaction, the sulfur dioxide (SO2) and hydrogen chloride (HCl) generated are both gases that can leave the reaction vessel, driving the reaction forward. Excess thionyl chloride (b.p. 74.6 °C) is easily evaporated as well.[10] The reaction mechanisms involving thionyl chloride and phosphorus pentachloride are similar; the mechanism with thionyl chloride is illustrative:[11]
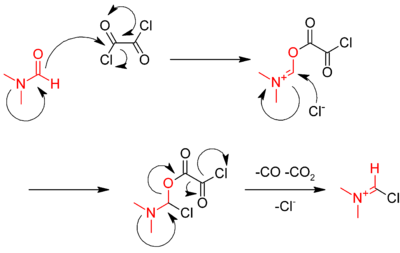
Another method involves the use of oxalyl chloride:
- RCOOH + ClCOCOCl → RCOCl + CO + CO2 + HCl
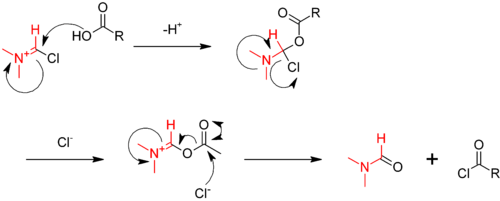
The reaction is catalysed by dimethylformamide (DMF), which reacts with oxalyl chloride in the first step to give the iminium intermediate.
The iminium intermediate reacts with the carboxylic acid, abstracting an oxide, and regenerating the DMF catalyst.[11]
Finally, methods that do not form HCl are also known, such as the Appel reaction:[12]
- RCOOH + Ph3P + CCl4 → RCOCl + Ph3PO + HCCl3
and the use of cyanuric chloride (C3N3Cl3):[13]
Reactions
Nucleophilic reactions
Acyl chlorides react with water yielding the carboxylic acid:
- RCOCl + H2O → RCO2H + HCl
Acyl chlorides are used to prepare acid anhydrides, esters, and amides by reacting acid chlorides with: a salt of a carboxylic acid, an alcohol, or an amine, respectively. The use of a base, e.g. aqueous sodium hydroxide or pyridine,[10] or excess amine (when preparing amides)[11] is desirable to remove the hydrogen chloride byproduct, and to catalyze the reaction. While it is often possible to obtain esters or amides from the carboxylic acid with alcohols or amines, the reactions are reversible, often leading to low yields. In contrast, both reactions involved in preparing esters and amides via acyl chlorides (acyl chloride formation from carboxylic acid, followed by coupling with the alcohol or amine) are fast and irreversible. This makes the two-step route often preferable to the single step reaction with the carboxylic acid.[10]
With carbon nucleophiles such as Grignard reagents, acyl chlorides generally react first to give the ketone and then with a second equivalent to the tertiary alcohol. A notable exception is the reaction of acyl halides with certain organocadmium reagents which stops at the ketone stage. The nucleophilic reaction with Gilman reagents (lithium diorganocopper compounds) also afford ketones, due to their lesser reactivity.[10] Acid chlorides of aromatic acids are generally less reactive those of alkyl acids and thus somewhat more rigorous conditions are required for reaction.
Acyl chlorides are reduced by lithium aluminium hydride and diisobutylaluminium hydride to give primary alcohols. Lithium tri-tert-butoxyaluminium hydride, a bulky hydride donor, reduces acyl chlorides to aldehydes, as does the Rosenmund reduction using hydrogen gas over a poisoned palladium catalyst.[14]
Electrophilic reactions
With Lewis acid catalysts like ferric chloride or aluminium chloride, acyl chlorides participate in Friedel-Crafts acylations, to give aryl ketones:[8][10]
Because of the harsh conditions and the reactivity of the intermediates, this otherwise quite useful reaction tends to be messy, as well as environmentally unfriendly.
Hazards
Low molecular weight acyl chlorides are often lachrymators, and they react violently with water, alcohols, and amines.
References
- Nomenclature of Organic Chemistry, R-5.7.6 Acid halides
- Sih, John C. (2001-04-15), "Formyl Chloride", in John Wiley & Sons, Ltd (ed.), Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, doi:10.1002/047084289x.rf026, ISBN 9780471936237
- Richard O.C. Norman; James M. Coxon (16 September 1993). Principles of Organic Synthesis, 3rd Edition. CRC Press. p. 371. ISBN 978-0-7487-6162-3.
- US patent 5672749, Phillip R. DeVrou, W. Bryan Waites, Robert E. Young, "Process for preparing acetyl chloride"
- Samel, Ulf-Rainer; Kohler, Walter; Gamer, Armin Otto; Keuser, Ullrich (2005). "Propionic acid and derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a22_223.
- Maki, Takao; Takeda, Kazuo (2002). "Benzoic acid and derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_555.
- Helferich, B.; Schaefer, W. (1929). "n-Butyrl chloride". Organic Syntheses. 9: 32. doi:10.15227/orgsyn.009.0032.
- Allen, C. F. H.; Barker, W. E. (1932). "Desoxybenzoin". Organic Syntheses. 12: 16. doi:10.15227/orgsyn.012.0016.
- Adams, Roger (1923). "p-Nitrobenzoyl chloride". Organic Syntheses. 3: 75. doi:10.15227/orgsyn.003.0075.
- Boyd, Robert W.; Morrison, Robert (1992). Organic chemistry. Englewood Cliffs, N.J: Prentice Hall. pp. 666–762. ISBN 0-13-643669-2.
- Clayden, Jonathan (2001). Organic chemistry. Oxford: Oxford University Press. pp. 276–296. ISBN 0-19-850346-6.
- "Triphenylphosphine-carbon tetrachloride Taschner, Michael J. e-EROS: Encyclopedia of Reagents for Organic Synthesis, 2001
- K. Venkataraman; D. R. Wagle (1979). "Cyanuric chloride : a useful reagent for converting carboxylic acids into chlorides, esters, amides and peptides". Tetrahedron Lett. 20 (32): 3037–3040. doi:10.1016/S0040-4039(00)71006-9.
- William Reusch. "Carboxylic Acid Derivatives". VirtualText of Organic Chemistry. Michigan State University. Archived from the original on 2016-05-16. Retrieved 2009-02-19.