Sugarcane grassy shoot disease
Sugarcane grassy shoot disease (SCGS), caused by small, parasitic bacteria, contributes to losses of 5% to 20% in the main crop of sugarcane, and these losses are higher in the ratoon crop. A higher incidence of SCGS has been recorded in some parts of Southeast Asia and India, resulting in 100% loss in cane yield and sugar production.[1][2][3]
| Sugarcane grassy shoot disease | |
|---|---|
| Common names | SCGS, Sugarcane grassy shoot phytoplasma, grassy shoot of sugarcane |
| Causal agents | Phytoplasma |
| Hosts | sugarcane |
| Vectors | leafhoppers (Saccharosydne saccharivora, Matsumuratettix hiroglyphicus,Deltocephalus vulgaris and Yamatotettix flavovittatus) |
| EPPO Code | PHYP48 |
| Distribution | Southeast Asia and India |
Causal organism and transmission
Causal organism
- SCGS disease is caused by a phytoplasma (Candidatus phytoplasma), which is one of the destructive pathogens of sugarcane (Saccharum officinarum L). In India, SCGS phytoplasmas are spreading at an alarming rate, adversely affecting yield of the sugarcane crop.[1][2][3]
- Phytoplasmas, formerly called mycoplasma-like organisms (MLOs), are a large group of obligate, intracellular, cell wallless parasites classified within the class Mollicutes.[2][3]
- Phytoplasmas are associated with plant diseases and are known to cause more than 600 diseases in several hundred plant species, including gramineous weeds and cereals.[4][5][6][7][8][9][10] The symptoms shown by infected plants include: whitening or yellowing of the leaves, shortening of the internodes (leading to stunted growth), smaller leaves and excessive proliferation of shoots, resulting in a broom phenotype and loss of apical dominance.[8]
Transmission
Sugarcane is a vegetatively propagated crop, so the pathogen is transmitted via seed material and by phloem-feeding leafhopper vectors.[11][12] Saccharosydne saccharivora,[13] Matsumuratettix hiroglyphicus,[14] Deltocephalus vulgaris[15] and Yamatotettix flavovittatus[16] have been confirmed as vectors for phytoplasma transmission in sugarcane. Unconfirmed reports also suggest a spread through the steel blades (machetes) used for sugarcane harvesting..

SCGS disease symptoms[1]
Phytoplasma-infected sugarcane plants show a proliferation of tillers, which give it typical grassy appearance, hence the name grassy shoot disease. The leaves of infected plants do not produce chlorophyll, and therefore appear white or creamy yellow. The leaf veins turn white first as the phytoplasma resides in leaf phloem tissue. Symptoms at the early stage of the plant life cycle include leaf chlorosis, mainly at the central leaf whorl. Infected plants do not have the capacity to produce food in the absence of chlorophyll, which results in no cane formation. These symptoms can be seen prominently in the stubble crop. The eye or lateral buds sprout before the normal time on growing cane. A survey of various fields of western Maharashtra showed grassy shoot with chlorotic or creamy white leaves was the most prevalent phenotype in sugarcane plants infected with SCGS.

SCGS and sugarcane iron deficiency

Symptoms of iron deficiency (interveinal chlorosis) are very similar to those of SCGS. It shows creamy leaves, but no chlorosis occurs in leaf veins, and they remain green. In the case of severe iron deficiency, veins may lose chlorophyll in the absence of iron and appear similar to SCGS disease.[17][18] Iron deficiency is caused by a lack of iron nutrients in the soil; therefore, one may observe several plants showing symptoms of iron deficiency in localized patches in a field. Phytoplasma-infected plants, though, may occur anywhere in the field in a more random distribution. Treatment with 0.1% ferrous sulfate, either by spraying or supplying it through fertilizer cures iron deficiency, but phytoplasma-infected sugarcane does not respond to any treatment. Phytoplasma-infected plants growing in vitro show sensitivity to tetracycline.[17][18]
Detection methods
Based on symptoms[1]
Phytoplasma-infected sugarcane can be recognized by visual symptoms, but there are limitations.[1]
- Visual symptoms occur only after considerable growth, normally two to three weeks after planting.[1]
- If not observed keenly, confusion may occur on differences between symptoms of SCGS disease and iron deficiency.
- In addition to above points, the poor relationship between symptoms and phytoplasma presence has been confirmed by earlier findings that symptoms alone are not reliable indicators of infection or identity. This highlights the importance of employing tests, such as the molecular tests, to verify associations between phytoplasma and putative disease symptoms. Also it suggests the inability to recognize a symptomless sugarcane harboring a phytoplasma could result in inadvertent exposure of sugarcane to a potential disease source.[19]
- Precise diagnosis is therefore necessary for effective disease identification and control. Though reliable, DNA hybridization,[20] electron microscopy[12] and PCR[21] techniques require specialized equipment and trained human resources. Among these, PCR is an accurate, economical and convenient method, which allows analysis of samples in a short time.
Polymerase chain reaction
In recent years, regions of the rRNA operon of the prokaryotic and eukaryotic organisms have been sequenced and are being used to develop PCR-based detection assays. These sequences are highly specific to the infecting organism.
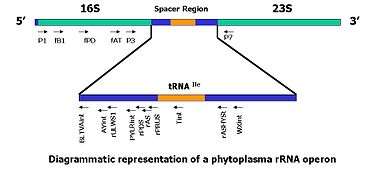
- The ribosomal DNA contains one transcriptional unit with a cluster of genes coding for the 18S, 5.8S and 28S rRNAs and two internal transcribed spacer regions, ITS1 and ITS2[22] in eukaryotes, and for 16S, 5S and 23S in prokaryotes . Previous studies have demonstrated the complex ITS regions are useful in measuring close genealogical relationships, because they exhibit greater interspecies differences than the smaller and larger subunits of rRNA genes.[10]
- The use of specific probes as selective PCR primers offers an impressive approach for the rapid identification of a large number of phytoplasma isolates.[1][10][21][23][24][25]
- The agarose gel electrophoresis image beside shows amplification of partial, 1.2kb long, 16S rDNA] of Candidatus phytoplasma, where total genomic DNA from a suspected sugarcane plant has been used as template DNA.[1]
ELISA
ELISA has been used as a diagnostic tool in medicine and plant pathology. Serological detection using a specific antiserum is an economical and convenient method that allows for both analysis of many samples in a short time and immunohistological observation of infected tissues.
- A phytoplasma-specific antibody can be prepared using the following steps:
- Cloning of the phytoplasma gene fragment into an Escherichia coli expression vector;
- Expression in E. coli and purification of the protein; and
- Preparation of antibodies in rabbits.
Control
In SCGS disease, the primary concern is to prevent the disease rather than treat it. Large numbers of phytoplasma-infected seed sets used by the farmers usually cause fast SCGS disease spread. Healthy, certified 'disease free' sugarcane sets are suggested as planting material. If disease symptoms are visible within two weeks after planting, such plants can be replaced by healthy plants. Uprooted infected sugarcane plants need to disposed of by burning them.
Moist hot air treatment of sets is suggested to control infection[29] before planting. This reduces percentage of disease incidence, but causes a reduction in the percentage of bud sprouting.
Reports that the disease spreads through steel blades used for sugarcane harvesting are unconfirmed, but treating the knives using a disinfectant (Lysol) or by dipping them in boiling water for some time is suggested as a precaution.
Phytoplasma infection also spreads through insect vectors; it is therefore important to control them.
- General field observation reports the ratoon crop has higher percentage of disease incidence than the initial planted (main) crop. When the disease incidence is more than 20%, it is suggested to discontinue that crop cycle.
- It is always wise to purchase the certified planting material from authorized seed growers, which assures disease-free planting material.
References
- Nasare, K., Yadav, Amit., Singh, A. K., Shivasharanappa, K. B., Nerkar, Y. S., and Reddy, V. S. Molecular and symptom analysis reveal the presence of new phytoplasmas associated with sugarcane grassy shoot disease in India. (2007). Plant Disease. 91:1413-1418..
- Rao, G. P. and Ford, R. E. (2000) Vectors of virus and Phytoplasma diseases of Sugarcane: An Overview. In: Sugarcane Pathology, Vol. III. Virus and Phytoplasma diseases, G.P. Rao, R.E. Ford, M. Tosic and D.S. Teakle (Eds) Science Publishers, Hamshere, USA, Pg: 265-314.
- Rao, G. P. and Dhumal, K. N. (2002) Grassy Shoot Disease of Sugarcane. In: Sugarcane Crop Management by Singh S., Rao G. and Easwatnamoorthy S. SCI TECH Publications, USA. Pg. 208-222.
- Kakizawa, S., Oshima, K. and Namba, S. (2006) Diversity and Functional importance of Phytoplasma membrane proteins. Trends in Microbiology. 14: 254-256.
- Kakizawa, S., Oshima, K., Kubuyama, T., Nishigava, H., Jung, H., Sawayanagi, T., Tsuchizaki, T., Miyata, S., Ugaki, M. and Namba, S. (2001) Cloning and Expression analysis of Phytoplasma protein translocation genes. MPMI. 9: 1043-1050.
- Kakizawa, S., Oshima, K., Nishigawa, H., Jung, H., Wei, W., Suzuki S., Tanaka, M., Miyata, S., Ugaki, M. and Namba, S. (2004) Secretion of immunodominant membrane protein from onion yellows phytoplasma through the Sec protein-translocation system in E. coli. Microbiology. 150: 135-142.
- Kakizawa, W., Jung, S., Suzuki H., Tanaka, M., Nishigawa, H., Miyata, S., Oshima, K., Ugaki, M. and Namba, S. (2004) An antibody against the SecA membrane protein of one Phytoplasma reacts with those of phytogenetically different phytoplasmas. Phytopathology. 94: 683-686.
- Lee, I. M., Davis, R. E., and Gundersen-Rindal, D.E. (2000) Phytoplasma: Phytopathogenic mollicutes. Annu. Rev. Microbiol. 54: 221-255.
- Lee, I. M., Davis, R. E., and Gundersen-Rindal, D.E. and Bertaccini A. (1998) Phytoplasma: Ecology and Genomic Diversity. Phytopathology. 88:1359-1366.
- Lee, I.M., Hammond, R.W., Davis, R.E. and Gunderson D.E. (1993) Universal amplification and analysis of pathogen 16SrDNA for classification and Identification of Mycoplasma like Organisms. Molecular Plant Pathol. 83(8): 834-842.
- Shomi, T. and Sugiura, M. Grouping of mycoplasma-like organisms transmitted by the leafhopper vector, Macrosteles orientalis Virvaste, based on host range. (1984). Ann. Phytopatholol. Soc. Jpn. 50: 149-157.
- Kavakita, H., Saiki, T., Mitsuhashi, W., Watanabe, K. and Sato, M. (2000) Identification of Mulberry Dwarf Phytoplasma in the genital and eggs of leafhopper Hishimonoides sellatiformis. Bateriology. 90: 909-914.
- Arocha, Y., Lopez, M., Fernandez, B., Pinol, D., Horta, D., Peralta, R., Almeida O., Picornell, S., Wilson, M and Jones P (2005) Transmission of a Sugarcane Yellow Leaf Phytoplasma by the Delphacid Plant hopper Saccharosydne saccharivora, a new vector of sugarcane yellow leaf syndrome. Plant Pathology. 54: 634-642.
- SHanboonsong Y., Choosai C., Panyim S. and Damak S. (2002). Transovarial transmission of sugarcane white leaf phytoplasma in the insect vector Matsumuratettix hiroglyphicus (Matsumura) . Insect Molecular Biology. 11(1): 97–103
- Srivastava, S., Singh, V., Gupta, P. S., Sinha O. K., and Baitha, A. Nested PCR assay for detection of sugarcane grassy shoot phytoplasma in the leafhopper vector Deltocephalus vulgaris a first report. (2006). Plant Pathol. 22:25-28.
- HANBOONSONG Y., RITTHISON W., CHOOSAI C., SIRITHORN P. Transmission of sugarcane white leaf phytoplasma by Yamatotettix flavovittatus, a new leafhopper vector (2006). Journal of Economic Entomology. 99(5):1531-1537. ISSN 0022-0493
- Dametie, T. Mamo, A. Zelleke (1995) Studies on Iron Chlorosis of Sugar Cane (Saccharum officinarum L.) at Metahara, Ethiopia: Soil and Plant Characterisation and Efficiency of Different Iron Sources. Journal of Agronomy and Crop Science 175 (5), 317-324.
- Pal R., D. P. Motiramani, S. B. Gupta, and B. S. Bhargava.(1990) Chlorosis in sugarcane: Associated soil properties, leaf mineral composition, and crop response to iron and manganese. Journal Nutrient Cycling in Agroecosystems 22 (3) 129-136.
- Blanche, K. R., Tran-Nguyen, T. T., and Gibb, K. S. 2003. Detection, identification and significance of phytoplasmas in grasses in northern Australia. Plant Pathol. 52:505-512.
- Webb, D.R., Bonfiglioli, R.G., Carraro, L., Osler, R. and Symons, R.H. Oligonucleotide as Hybridization probes to Localize Phytoplasmas in Host Plants and Insect Vectors. (1998). Phytopathology. 89: 894-901.
- Smart, C. D., Schneider, B., Blomquist, C., Guerra, L. J., Harrison, N. A., Ahrens, U., Lorenz, K. H., Seemüller, E., and Kirkpatrick, B. C. Identification of phytoplasma strain-specific PCR primers obtained from 16S/23S rRNA spacer sequences. (1996). Appl. Environ. Microbiol. 62: 2988-2993.
- White, T. J., T. Bruns, S. Lee, and J. W. Taylor. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR Protocols: A Guide to Methods and Applications, eds. Innis, M. A., D. H. Gelfand, J. J. Sninsky, and T. J. White. Academic Press, Inc., New York. Pp. 315-322.
- Namba, S., Kato S., Iwanami, S., Oyaizu, H., Shizawa, H. and Tsuchizaki, T. (1993) Detection and differentiation of Plant-Pathogenic Mycoplasma like organisms using Polymerase Chain Reaction. Phytopathology. 83: 786-791.
- Ahrens, U., and Seemüller, E. (1992) Detection of DNA of plant pathogenic mycoplasma-like organisms by a polymerase chain reaction that amplifies a sequence of the 16S rRNA gene. Phytopathology. 82:828-832.
- Gundersen, D., Lee, I. M., Rehner, S., Davis, R. and Kingsbury D. (1994) Phylogeny of Mycoplasmalike organisms (Phytoplasmas): a basis for their classification. Journal of Bacteriology. 176: 5244-5254.
- An antigenic protein gene of phytoplasma associated with sweet potato witche’s broom. (1998). Yu, Y., Yeh, K. and Lin, C. Microbiology. 144: 1257-1262.
- Berg, M., Davies, D., Clark, M., Vetten, H., Maier, G., Marcone, C. and Seemuller E. (1999) Isolation of gene encoding an immunodominant membrane protein of the apple proliferation phytoplasma and expression and characterization of the gene product. Microbiology. 145: 1937-1943.
- Chen, Y. and Chen, T. (1998) Expression of engineered antibodies in plans: A possible tool for Spiroplasma and Phytoplasma disease control. Phytopathology. 88: 1367-1371.
- Vishwanathan, R. Grassy shoot. In: A Guide to Sugarcane Diseases. P. Rott, R.A. Bailey, J.C. Comstock, B.J. Croft and A.S. Saumtally, (Eds) France: CIRAD ISSCT. (2000). Pg: 215-220.
External links
| Wikimedia Commons has media related to Saccharum. |
| Look up sugarcane grassy shoot disease in Wiktionary, the free dictionary. |
Further reading
- Sugarcane diseases; Authors: A. Sivanesan, J. M. Waller, Commonwealth Mycological Institute by CAB International (1986).
- A guide to sugarcane diseases; Authors:Philippe Rott, R.A. Bailey, A.S. Saumtally, International Society of Sugar Cane Technologists (ISSCT), 1999.
- Sugarcane Pathology: Virus and Phytoplasma Diseases; Authors: G.P. Rao, R.E. Ford, M. Tosic, and D.S. Teakle (eds.), ISBN 978-1-57808-128-8; 2001.
- Vasantdada Sugar Institute, Manjari Village, Pune, Maharashtra, India
- Sugarcane Breeding Institute, Coimbatore, Tamilnadu, India
- Indian Institute of Sugarcane Research, Lucknow, Uttar Pradesh, India