SQ109
SQ109 is a drug undergoing development for treatment of tuberculosis.[1][2]
 | |
| Names | |
|---|---|
| IUPAC name
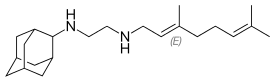
N-Adamantan-2-yl-N'-((E)-3,7-dimethyl-octa-2,6-dienyl)-ethane-1,2-diamine | |
| Identifiers | |
3D model (JSmol) |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C22H38N2 | |
| Molar mass | 330.560 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Background
On October 16, 2007, it was given the status of Orphan drug by the U.S. Food and Drug Administration (FDA) for use against drug-susceptible and drug-resistant TB bacteria.[3]
SQ109 completed three phase I studies in the U.S. and one Phase II efficacy studies in tuberculosis patients in Africa. SQ109 showed activity against both drug susceptible and multi-drug-resistant tuberculosis bacteria, including extensively drug-resistant tuberculosis strains. In preclinical studies SQ109 enhanced the activity of anti-tubercular drugs isoniazid and rifampin and reduced by >30% the time required to cure mice of experimental TB.
SQ109 is being developed by OOO Infectex in Russia and by Sequella Inc internationally. In July 2012, Infectex received notification from the Russian Ministry of Health for approval to begin the pivotal clinical trial associated with a drug registration submission, and can proceed with the clinical development of SQ109 for treatment of tuberculosis in the Russian Federation.[4]
See also
- Adamantane
- Ethambutol (An older drug with a similar mechanism of action)
References
- Jia, L.; Tomaszewski, J. E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M. (2009). "Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug". British Journal of Pharmacology. 144 (1): 80–87. doi:10.1038/sj.bjp.0705984. PMC 1575972. PMID 15644871.
- Meng, Q.; Luo, H.; Liu, Y.; Li, W.; Zhang, W.; Yao, Q. (2009). "Synthesis and evaluation of carbamate prodrugs of SQ109 as antituberculosis agents". Bioorganic & Medicinal Chemistry Letters. 19 (10): 2808–10. doi:10.1016/j.bmcl.2009.03.091. PMID 19362471.
- "New FDA Orphan Drugs: AVI-4658, SQ109, ATIR". Medscape Medical News. 11 November 2007.
- "PRESS RELEASE Maxwell Biotech Venture Fund`s Portfolio Company, Infectex, Receives Russian Regulator`s Approval to Conduct Pivotal Clinical Trial for Sequella's Antibiotic, SQ109, for Tuberculosis". Reuters. 26 July 2012.