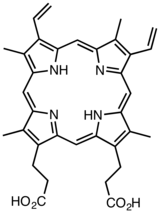
Protoporphyrin IX
Protoporphyrin IX is an organic compound, specifically a porphyrin, that plays an important role in living organisms as a precursor to other critical compounds like hemoglobin and chlorophyll. It is a deeply colored solid that is not soluble in basic water. The name is often abbreviated as PPIX.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.213 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C34H34N4O4 | |
| Molar mass | 562.658 g/mol |
| Density | 1.27 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The Protoporphyrin IX molecule contains the porphine core, a tetrapyrrole macrocycle that shows marked aromatic character. The molecule is essentially planar, except for the N-H bonds that are bent out of the plane of the rings, in opposite (trans) directions.[1]
The general term protoporphyrin refers to porphine derivatives that have the outer hydrogen atoms in the four pyrrole rings replaced by four methyl groups −CH
3 (M), two vinyl groups −CH=CH
2 (V), and two propionic acid groups −CH
2−CH
2−COOH (P). The Roman numeral "IX" indicates that these chains occur in the circular order MV-MV-MP-PM around the outer cycle. (The numbering of the variants is traditional and not entirely systematic.)
Natural occurrence
The compound is encountered in nature in the form of complexes where the two inner hydrogen atoms are replaced by a divalent metal cation. When complexed with an iron(II) (ferrous) cation Fe2+, the molecule is called heme. Hemes are prosthetic groups in some important proteins. These heme-containing proteins include hemoglobin, myoglobin, and cytochrome c. Complexes can also be formed with other metal ions, such as zinc.[2]
Biosynthesis
The compound is synthesized from acyclic precursors via a mono-pyrrole (porphobilinogen) then a tetrapyrrole (a porphyrinogen, specifically uroporphyrinogen III). This precursor is converted to protoporphyrinogen IX, which is oxidized to protoporphyrin IX.[2]The last step is mediated by the enzyme protoporphyrinogen oxidase.
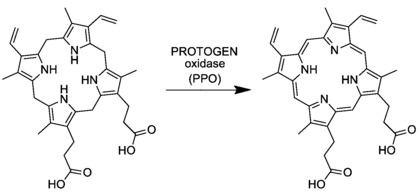
Protoporphyrin IX is an important precursor to biologically essential prosthetic groups such as heme, cytochrome c, and chlorophylls. As a result, a number of organisms are able to synthesize this tetrapyrrole from basic precursors such as glycine and succinyl CoA, or glutamate. Despite the wide range of organisms that synthesize protoporphyrin IX the process is largely conserved from bacteria to mammals with a few distinct exceptions in higher plants.[3][4][5]
In the biosynthesis of those molecules, the metal cation is inserted into protoporphyrin IX by enzymes called chelatases. For example, ferrochelatase converts the compound into heme b (i.e. Fe-protoporphyrin IX or protoheme IX). In chlorophyll biosynthesis, the enzyme magnesium chelatase converts it into Mg-protoporphyrin IX.
Synthetic iron derivatives
Protoporphyrin IX reacts with iron salts in air to give the complex FeCl(PPIX).[6]
See also
References
- Winslow S. Caughey, James A. Ibers (1977). "Crystal and Molecular Structure of the Free Base Porphyrin, Protoporphyrin IX Dimethyl Ester". J. Am. Chem. Soc. 99: 6639–6645. doi:10.1021/ja00462a027.CS1 maint: uses authors parameter (link)
- Paul R. Ortiz de Montellano (2008). "Hemes in Biology". Wiley Encyclopedia of Chemical Biology. John Wiley & Sons. doi:10.1002/9780470048672.wecb221.
- A. R. Battersby; C. J. R. Fookes; G. W. J. Matcham; E. McDonald (1980). "Biosynthesis of the pigments of life: formation of the macrocycle". Nature. 285: 17–21. doi:10.1038/285017a0.
- F. J. Leeper (1983). "The biosynthesis of porphyrins, chlorophylls, and vitamin B12". Natural Product Reports. 2: 19–47. doi:10.1039/NP9850200019.
- G. Layer; J. Reichelt; D. Jahn (2010). "Structure and function of enzymes in heme biosynthesis". Protein Science. 19: 1137–1161. doi:10.1002/pro.405. PMC 2895239. PMID 20506125.
- Chang, C. K.; DiNello, R. K.; Dolphin, D. (1980). "Iron Porphines". Inorg. Synth. Inorganic Syntheses. 20: 147. doi:10.1002/9780470132517.ch35.